6. DISEASE MANAGEMENT IN MALES
6.1. Conservative options
6.1.1. Observation
A stricture will usually result in diminution in flow once the calibre of the urethral lumen is < 10 Fr [144]. In other strictures (> 10 Fr), the diagnosis is often made by coincidence in asymptomatic patients because of a urologic examination for other reasons (e.g., cystoscopy, need for urethral catheterisation) [144]. Purohit et al., performed observation and repeated cystoscopic evaluation of 42 subclinical, incidentally encountered strictures (> 16 Fr). After a median follow-up of 23 months, only five (12%) strictures progressed to a low-grade stricture (11-15 Fr). No patient developed symptoms and none of them needed surgical intervention [144]. These patients are candidates for observation although no evidence exist on the long-term evolution of these strictures.
In a series of anatomic stricture recurrence (< 16 Fr) after urethroplasty, only 65% of patients were symptomatic [145]. Some asymptomatic patients refused further intervention because they had experienced substantial improvement after their primary urethroplasty. These patients were considered as functional “successes” [145]. A multicentric study of the Trauma and Urologic Reconstructive Network of Surgeons observed an important discrepancy between cystoscopic recurrence and need for further intervention [143]. Patients with a large calibre (> 16 Fr) recurrence had a one and two-year need for intervention rate of 4% and 12%, respectively. Of note, patients with small-calibre (< 16 Fr) recurrence had a one and two-year need for intervention rate of only 41% and 49%. Patients who needed intervention had poorer PROMs suggesting clinical symptoms and bother. There is no information on long-term complications in patients with recurrences who did not undergo intervention. In cases of an asymptomatic stricture recurrence, it might be an option not to intervene but to perform regular follow-up.
Care must be taken about the term “asymptomatic” stricture (recurrence) as patients might conceal their bother and symptoms by different means (not drinking, social avoidance) and might only search for medical help once concealment is no longer tenable [190].
6.1.2. Suprapubic catheter
Radiation-induced urethral strictures are a difficult to treat population as stricture-free rates for urethral reconstruction are lower compared to those in non-irradiated patients [191]. Fuchs et al., evaluated 75 patients who were initially treated by suprapubic diversion for radiation-induced isolated BMS [192]. Only 51% eventually decided to undergo urethroplasty after a mean follow-up period of 25 months. Although there was no significant difference in overall performance status between patients with a chronic suprapubic catheter vs. those undergoing urethroplasty, all patients with a poor performance score remained with a suprapubic catheter. Patients with concomitant stress urinary incontinence (SUI) opted more often to keep their suprapubic catheter as the SUI improved in 61% of cases. On the other hand, patients who kept their suprapubic catheter suffered from catheter-related complications in 27% of cases. Urinary diversion by ileal conduit was performed in 30% of patients who remained with a suprapubic catheter while this was only the case in 8% who underwent urethroplasty. A suprapubic catheter is also an option in frail patients not able to undergo surgery or in patients who do not want (further) urethral surgery and are willing to accept the complications of a suprapubic catheter [193].
| Summary of evidence | LE |
| Patients with asymptomatic incidental (> 16 Fr) strictures have a low risk of progression and to develop symptoms. | 3 |
| Only half of the patients initially treated with a suprapubic catheter for radiation-induced bulbomembranous strictures will proceed with urethroplasty. | 3 |
| Recommendations | Strength rating |
| Do not intervene in patients with asymptomatic incidental (> 16 Fr) strictures. | Weak |
| Consider long-term suprapubic catheter in patients with radiation-induced bulbomembranous strictures and/or poor performance status. | Weak |
6.2. Endoluminal treatment of anterior urethral strictures in males
The ability to treat the majority of strictures by less invasive and time-consuming means, offers obvious benefits particularly when specialist surgical services are not available, or patients simply prefer a more pragmatic immediately available solution.
6.2.1. Direct vision internal urethrotomy
In contemporary practice, direct vision internal urethrotomy (DVIU) is commonly performed as a first-line treatment of urethral strictures [194]. It is usually performed under general or spinal anaesthesia in well-resourced countries but shown to be well tolerated under local anaesthesia with or without sedation [195].
6.2.1.1. Indications of “cold knife” direct vision internal urethrotomy
6.2.1.1.1. Direct vision internal urethrotomy for primary stricture treatment
In the only high-level evidence study, Steenkamp et al., randomised 210 patients with seemingly comparable non-obliterative strictures at all locations of the urethra to either filiform dilatation vs. DVIU with local anaesthesia on an outpatient basis [196]. They collected objective data with RUG performed at seven follow-up visits (3, 6, 9, 12, 24, 36 and 48 months). This unique study showed that urethral dilatation is equally effective as DVIU but both procedure modalities become less effective with increasing stricture length (see section 6.2.1.1.3.1).
A retrospective cohort series on the primary treatment of patients with iatrogenic urethral strictures reported a significantly poorer patency rate for DVIU compared to urethroplasty techniques (32.2 versus 82.4-83.5%) [197].
Patency rates vary considerably between 8% and 77% after DVIU (predominantly without prior urethroplasty) in retrospective cohort studies with minimum follow-up of one year [67,198-204] (Table 6.1). Median time to recurrence was less than twelve months in most series [67,198-202]. This large variation in patency rate can be in part explained by the heterogeneous nature of the strictures and various definitions of patency used by the authors in these series. Indication to perform DVIU is dependent on various stricture characteristics that are prognostic for a successful outcome.
Table 6.1: Results of DVIU in series with minimum follow-up > 12 months
| Study | N | Age (years) | Follow-up (months) | Location | Length (cm) | Previous interventions | TTR (months) | Patency rate (%) |
| Al Taweel et al. [200] | 301 | 37 (range: 17-82) | 36 | Bulbar: 227 (75%) | 1.3 (0.4-4.2) | Primary: 47% | 10 | 8.3 |
| Penile: 50 (17%) | ||||||||
| Recurrent: 53% | - | - | ||||||
| Penobulbar: 24 (8%) | ||||||||
| Barbagli et al. [199] | 136 | 37 (IQR: 25-48) | 55 (range: 36-92) | Bulbar: 100% | 1-2 cm: 45% | Primary: 100% | 25 | 57 |
| 2-3 cm: 40% | ||||||||
| 3-4 cm: 15% | ||||||||
| Kluth et al. [198] | 128 | 64 (SD: 16) | 16 (IQR: 6-43) | Penile: 15 (12) | NR | Primary: 66% | 8 | 52 |
| Bulbar: 112 (88) | ||||||||
| Recurrent: 34% | - | - | ||||||
| Unknown: 1 (1%) | ||||||||
| Pal et al. [201] | 186 | 39 (SD:15) | 1st DVIU: 58 (SD: 15) | Bulbar: 100% | NR | Primary: 69% | 8.5 | 1st DVIU: 30 |
| 2nd DVIU: 56 (SD: 15) | ||||||||
| Repeat: 31% | - | 2nd DVIU: 23 | ||||||
| 3rd DVIU: 45 (SD: 15) | ||||||||
3rd DVIU: 13 | ||||||||
| Launonen et al. [202] | 34 | 6 (range: 0-16) | 79 (range: 7-209) | Bulbar: 74% | < 2 cm: 85% | Primary: 100% | 4 | 26% |
| Penile: 21% | > 2 cm: 15% | |||||||
| Penobulbar: 6% | - | |||||||
| Redon-Galvez et al. [203] | 67 | 57 (range: 15-91) | 40 (range: 12-120) | Penile: 9% | < 1 cm: 82% | Primary: 90% | < 24 | 63% |
| Bulbar: 64% | > 1 cm: 18% | Repeat: 10% | - | - | ||||
| VUA: 21% | ||||||||
| Membranous: 6% | ||||||||
| Güler Y. [197] | 234 | 57 (range: 22-74) | 47 (range: 24-56) | Penile: 34% | 2.5 (0.4-5) | Primary: 100% | - | 34% |
| Bulbar: 59% | 30% | |||||||
| Membranous: 6% | 33% | |||||||
| Harraz et al. [204] | 430 | 50 (SD: 15) | 29 (range: 3-132) | Bulbar: 100% | < 2 cm | NR, prior urethroplasty excluded | NR | 58% |
| Yürük et al. [67] | 193 | 65 (SD: 13) | 36 (SD: 12) | Bulbar: 100% | < 1 cm: 140 (73%) | 0% | 87% of recurrence < 3 | 77% |
| 1-2 cm: 21 (11%) | - | 100% of recurrence < 6 | - | |||||
| 2-3 cm: 32 (17%) |
DVIU = Direct vision internal urethrotomy; IQR = interquartile range; N = number of patients; NR = not reported; SD = standard deviation; TTR = time to recurrence.
6.2.1.1.2. Direct vision internal urethrotomy for recurrent strictures and as salvage treatment after failed urethroplasty
In the OPEN trial, a recurrent stricture was defined as at least one previous failed intervention (endoscopic urethrotomy, urethral dilatation, urethroplasty) [205]. The previous intervention was predominantly DVIU. Despite poor recruitment, 108 and 112 patients were randomised to urethroplasty and DVIU respectively in a 24-month study protocol. Both groups had a similar improvement in voiding score symptoms after intervention. However, patients undergoing urethroplasty had 2.6 higher odds of experiencing an improvement of > 10 ml/s in their maximum urinary flow compared to those undergoing urethrotomy (p=0.001) [205]. Need for re-intervention was observed in 13.8% vs. 25.9% of cases respectively allocated to urethroplasty and DVIU resulting in a 48% lower risk for re-intervention with urethroplasty (HR: 0.52; 95% CI: 0.31-0.89; p=0.017) [205]. Of note, self-dilatation was not considered a re-intervention [205]. Despite more re-interventions in the DVIU group, both treatments resulted in a similar improvement in quality of life but with a higher cost for urethroplasty with the limitation that the follow-up is only two years. [206]. Direct vision internal urethrotomy is also used as salvage treatment for recurrent strictures after urethroplasty. Brown et al., used DVIU for stricture recurrence (mean length: 4 cm; range: 1.5-7 cm) after excision and primary anastomosis (EPA), buccal mucosa grafts (BMG) urethroplasty and penile skin graft urethroplasty [207]. Patency was obtained in thirteen out of 37 cases (35%) after a single DVIU. After free graft urethroplasty (FGU), a short, veil-like stricture (or “diaphragm”) might develop at the distal or proximal end of the graft. Rosenbaum et al., used DVIU to a selected cohort of 43 patients with a short (< 1 cm), veil-like stricture after BMG urethroplasty [208]. After a mean follow-up of twelve months, patency rate was 51%.
6.2.1.1.3. Predictors of failure of “cold knife” direct vision internal urethrotomy
Several groups tried to identify prognostic factors to predict which patients are most likely to fail initial treatment (Table 6.2).
In the absence of well-designed, adequately powered multi-centre trials it is difficult to answer the question as to which clinical factors are predictive of failure of DVIU in men with urethral strictures. However, based on the predictors evaluated above, one can summarise that the best candidates are previously untreated patients with a single, short (max. 2 cm) bulbar stricture. Barbagli et al., reported a five-year patency rate of 71% for patients with untreated short (1-2 cm) bulbar urethral strictures [199].
Table 6.2: Predictors for urethral patency after direct vision internal urethrotomy
| Author | Location | Length | Calibre | Multiplicity | Prior DVIU |
| Steenkamp et al. [196] / Heyns [209] | RR for recurrence penile vs. bulbar: 1.85 (95% CI: 0.94 to 3.67, p = 0.077) | < 2 cm: 60% (@12 months) | NR | NR | None: 50-60% (@48 months) |
| - | 2-4 cm: 50% (@12m) | - | - | 1: 0-40% (@48 months) | |
| - | > 4 cm: 20% (@12 months) | - | - | 2: 0% (@24 months) | |
| Al Taweel et al. [200] | Bulbar: 11% | < 1 cm: 27% | NR | NR | 0: 12.1% |
| Penile: 0% | 1-2 cm: 0% | - | - | 1: 7.9% | |
| Penobulbar: 0% | > 2 cm: 0% | - | - | > 1: 0% | |
| Barbagli et al. [199] | NA | 1-2 cm: 71% (@60 months) | pQmax < 5 ml/s: 31% | NA | 0: 62% |
| - | 2-3 cm: 51% (@60 months) | pQmax 5-8 ml/s: 53% | - | 1: 37% | |
| - | 3-4 cm: 39% (@60 months) | pQmax > 8 ml/s: 83% | - | - | |
| Kluth et al. [198] | Location no predictor | NR | pQmax no predictor | NR | 0: 60% |
| - | - | - | - | > 1: 39% | |
| Pal et al. [201] | NA | < 1 cm: 45% | NR | Single: 35% | 0: 30% |
| - | 1-1.5 cm: 0% | - | Multiple: 0% | 1: 23% | |
| - | > 1.5 cm: 0% | - | - | 2: 13% | |
| Launonen [202] | Bulbar: 76%* | < 2 cm: 83%* | NR | NR | 0: 26% |
| Penile: 71%* | > 2 cm: 0%* | - | - | 1: 33% | |
| - | - | - | - | 2: 26% | |
| - | - | - | - | 3: 11% | |
| - | - | - | - | 4: 0% | |
| Redon-Galvez [203] | NR | < 1 cm: 71% | NR | NR | NR |
| - | > 1 cm: 25% | - | - | - | |
| Güler Y [197] | Bulbar: 30% | <1.85 cm: OR: 0.86 (95% CI: 0.74-0.99; p=0.042) | NR | NR | NR |
| Penile: 34% | |||||
| Membranous: 33% |
DVIU = Direct vision internal urethrotomy; NA = not applicable; NR = not reported; pQmax = pre-operative maximum urinary flow; RR: relative risk; OR: Odds ratio; CI: confidentiality interval.
*patency rates are reported after repetitive treatments.
6.2.1.2. Indications of “hot-knife” direct vision internal urethrotomy
6.2.1.2.1. Laser urethrotomy
Lasers available for urological applications, including Neodymium:YAG, Argon, Holmium:YAG, Potassium titanyl phosphate (KTP) and Tm:Yag, have been used for the treatment of urethral strictures. A SR identified four RCTs comparing laser urethrotomy and the “cold knife” urethrotomy. All studies were limited by short-term outcome evaluation and none of these four studies specified the results based on the location of the stricture. Two of these studies reported specific recurrence rates and meta-analysis showed a relative risk (RR) for recurrence of 0.55 (95% CI: 0.18-1.66; p=0.29), 0.39 (95% CI: 0.19-0.81; p=0.01) and 0.44 (95% CI: 0.26-0.75; p=0.003) in favour of laser urethrotomy after three, six and twelve months respectively [210]. Jin et al., performed a SR including 44 case series on laser urethrotomy or “cold knife” DVIU [211]. This included nineteen articles on laser urethrotomy and 25 articles on “cold knife” DVIU. The overall weighted average stricture-free rate was 74.9% (371/495) and 68.5% (1874/2735) for laser vs. “cold knife” DVIU, respectively (p=0.004). Although statistically significant, the results must be interpreted with caution because of heterogeneity and because no details are provided on follow-up duration. Specifically looking at first DVIU, laser and “cold knife” DVIU obtained a stricture-free rate of 58.6% and 42.7%, respectively and the difference was no longer statistically significant (p=0.09). At the bulbar urethra, laser and “cold knife” DVIU yielded a stricture-free rate of 52.9% and 60%, respectively (p=0.66) [211].
After publication of this SR, the EAU Guideline Panel scope search identified three additional RCTs [212-214]. In the RCT of Yenice et al., patients with a primary, bulbar stricture were randomised either to “cold knife” DVIU (n=29) or holmium:YAG laser urethrotomy (n=34). After twelve months follow-up, no significant difference in patency rate was identified (79% for “cold knife” DVIU vs. 68% for laser urethrotomy, p=0.3) [213]. In their RCT, Chen et al., reported a better patency rate after one year with laser (n=24) compared to “cold knife” (n=22) DVIU (respectively 88% vs. 18%; p < 0.05). However, after two years the benefit for laser disappeared and after five years both techniques showed a low patency rate: 9% for “cold knife” DVIU vs. 12% for laser DVIU
(p > 0.05) [212]. Gamal et al., randomised patients between “cold knife” DVIU (n=40) and Holmium:Yag laser DVIU (n=40). At one year, they found an equally effective improvement of maximum urinary flow in both groups.
6.2.1.2.2. Plasmakinetic (bipolar) urethrotomy
Cecen et al., conducted an RCT comparing plasmakinetic with “cold knife” DVIU (n=136) [215]. They reported patency rates for plasmakinetic and “cold knife” urethrotomy at nine months in respectively 86% and 70% of cases (p=0.025). At eighteen months, patency rates for plasmakinetic and “cold knife” urethrotomy were 63% and 67%, respectively (p=0.643) [215]. A prospective cohort study on primary strictures < 2 cm reported a patency rate at twelve months in 23/30 (77%) cases for plasmakinetic DVIU vs. 19/30 (63%) cases with “cold knife” DVIU (p=0.04) [216]. A retrospective case series (n=27) reported a 74% patency rate for short (1-2.5 cm) strictures after a mean follow-up of fourteen months [217]. They reported negligible blood loss during the procedure and no post-operative incontinence.
Based on the conflicting results described above and considering the heterogeneity of series and absence of long-term follow-up, overall, the available studies do not support the efficacy of one technique of DVIU over another. Given the similar complication rates between techniques (see section 6.2.1.3), no recommendation can be made in favour of one technique over another.
6.2.1.3. Complications of direct vision internal urethrotomy
6.2.1.3.1. Complications of “cold knife” direct vision internal urethrotomy
An overall complication rate of 6.5% was reported in a SR of Jin et al., based on twelve articles including 1,940 patients [211] (Table 6.3).
Notably, erectile dysfunction (ED) was reported in 5.3% of cases in this review [211]. In addition, Graversen et al., reported ED in eleven out of 104 (10.6%) patients [218]. This risk appears higher in strictures located in the penile urethra and, in addition to the poor patency rates, the use of DVIU in the penile urethra must be discouraged [218,219].
6.2.1.3.2. Complications of “hot knife” direct vision internal urethrotomy
The SR of Jin et al., reported a total complication rate of 11.8% (39/330) [211] (Table 6.3).
6.2.1.3.3. Complications of “cold knife” vs. “hot knife” direct vision internal urethrotomy
In a SR of RCTs comparing “cold knife” DVIU vs. laser DVIU, only 1/4 series reported complications [210].
In the laser group, an 8.9% complication rate was found due to contrast extravasation to the perineum and stricture recurrence. For the “cold knife” DVIU, a 15.5% complication rate was reported related to bleeding [210]. Two later RCT’s reported similar rates of urinary extravasation [212,213] and urinary incontinence (UI) [212] with both techniques.
The SR of retrospective case series of Jin et al., found no significant differences in the incidence rates of UI, urinary extravasation and UTI between laser and “cold knife” DVIU [211]. However, urinary retention and haematuria were more frequent with laser compared to “cold knife” DVIU [211]. Conversely, In the series of Yenice et al., haematuria was only reported after “cold knife” DVIU but not after laser DVIU (p=0.6) [213] (Table 6.3).
Table 6.3: Complications after “cold knife” DVIU vs. laser DVIU
| Study/Complication | “Cold knife” DVIU (%) | Laser DVIU (%) | p-value |
| Jin et al. [211] | |||
| Urinary extravasation | 2.9 | 3.1 | 0.938 |
| Urinary incontinence | 4.1 | 2.1 | 0.259 |
| Urinary tract infection | 2.1 | 2.7 | 0.653 |
| Urinary retention | 0.4 | 9 | < 0.0001 |
| Haematuria | 2 | 5.2 | 0.034 |
| Epididymitis | 0.5 | NR | NA |
| Fever | 2.3 | NR | NA |
| Scrotal abscess | 0.3 | NR | NA |
| Erectile dysfunction | 5.3 | NR | NA |
| Urinary tract irritation | NR | 11.4 | NA |
| Urinary fistula | NR | 1.5 | NA |
| Dysuria | NR | 5.1 | NA |
| Yenice et al. [213] | |||
| Urinary extravasation | 0 | 2.9 | 0.6 |
| Haematuria | 10 | 0 | |
| Chen et al. [212] | |||
| Urinary extravasation | 9.1 | 4.2 | 0.5 |
| Urinary incontinence | 4.5 | 4.2 | |
DVIU = direct vision internal urethrotomy; NA = not applicable; NR = not reported.
6.2.1.3.4. Complications of direct vision internal urethrotomy vs. dilatation
A Cochrane review found no significant differences for overall intra-operative complications (single dilatation vs. DVIU respectively 14% vs. 11%; RR: 0.75; 95 CI: 0.36-1.55) nor for individual complications (difficulty urinating, haematuria, false passage, pain, knotting/breaking/bending filiform leader) [196,220]. The low rate of false passage for both DVIU and dilatation (respectively 0.96 and 0.94%) might be explained by the systematic use of a filiform leader in both groups which was inserted endoscopically in the dilatation group followed by coaxial dilatators [196,220].
A small retrospective study comparing balloon dilatation (n=31) with DVIU (n=25) showed less urethral bleeding (6.5 vs. 32%; p=0.017) and UTI (3.2 vs. 24%; p=0.037) with balloon dilatation [221].
Apart from acute peri-operative complications described above, the stricture length and number of strictures were reported to increase after DVIU. Other authors mention that repeat urethral manipulations (DVIU and/or dilatation) can increase stricture complexity and delays time to urethroplasty [222].
6.2.1.3.5. Complications of “cold knife” direct vision internal urethrotomy vs. urethroplasty
The OPEN-trial reported adverse events of any type in 61% and 26.1% after urethroplasty (all types) and DVIU respectively [205]. In the urethroplasty group, mouth pain (related to oral mucosa graft [OMG] harvesting) and wound infection was noted as complication in respectively 14.6% and 4.9% of cases. Erectile dysfunction was 4.9% and 2.6% after urethroplasty and DVIU, respectively. Serious adverse events were reported in 8.5% and 8.7% after urethroplasty and DVIU respectively [205].
| Summary of evidence | LE |
| Direct vision internal urethrotomy performs poorly in penile strictures. Direct vision internal urethrotomy at the penile urethra might provoke venous leakage from the corpora cavernosa with subsequent risk of erectile dysfunction. | 1b |
| Increased stricture length is associated with higher risk of failure of direct vision internal urethrotomy (DVIU). | 1b |
| In selected patients with a primary, single, short (< 2 cm) and non-obliterative bulbar stricture, a five-year stricture-free rate of up to 77% can be expected. | 3 |
| Direct vision internal urethrotomy has a stricture-free rate of 51% if performed for a short, veil-like recurrent stricture after prior bulbar urethroplasty. | 3 |
| There is conflicting evidence that “hot knife” (laser, plasmakinetic) DVIU would be superior compared to “cold knife” DVIU after more than one year of follow-up. | 1a |
| Recommendations | Strength rating |
| Do not use direct vision internal urethrotomy (DVIU) for penile strictures. | Strong |
| Do not use DVIU/dilatation as solitary treatment for long (> 2 cm) segment strictures. | Strong |
| Perform DVIU/dilatation for a primary, single, short (< 2 cm) and non-obliterative stricture at the bulbar urethra. | Weak |
| Perform DVIU/dilatation for a short, veil-like recurrent stricture after prior bulbar urethroplasty. | Weak |
| Use either “hot” or “cold knife” techniques to perform DVIU depending on operator experience and resources. | Weak |
6.2.2. Single dilatation
6.2.2.1. Modalities of dilatation and results
Dilatation can be done in the office, under local anaesthesia and without complex resources [223,224]. With dilatation, the urethral mucosa at the stricture site is stretched and the scarring is disrupted. This is opposed to DVIU where the stricture is incised. However, both treatment modalities use the same principle to achieve urethral patency: a breach of the urethral mucosa at the site of the stricture in which re-epithelialisation should occur faster than wound contraction [220].
When dilators are used to dilate bulbar urethral strictures, considerable experience is required to avoid accidental perforation of the urethra at the level of the stricture. In order to reduce the risks (esp. false passage, spongiosal perforation, urethral bleeding) of “classic” blind dilatation with rigid sounds [224], other strategies have been developed and evaluated in which the dilatation is visually controlled after a guidewire has been inserted (Table 6.4).
Although no direct comparative studies of blind vs. visually controlled dilatation are available, several studies have reported a low complication rate with visually controlled modifications of dilatation. The recurrence rate largely varies between 23.5-64.5% (Table 6.4).
Table 6.4: Results of visually controlled dilatation
| Study | Technique | N | FU (mo) | recurrence | Definition of failure | Complications | |||
| Haematuria | False passage | Procedural failure | UTI | ||||||
Hosseini et al. [224] | Nelaton urethral catheters | 333 | 43 (36-52) | 138 (41.4%) | Need for additional intervention | 12 (3.6%) | 2 (0.6%) | NR | 15 (4.5%) |
| Kallidonis et al. [225] | Coaxial S-curved | 310 | 12 | 90 (33%) | No recurrence @1 yr with maximum one additional procedure | 11 (3.5%) | 0 (0%) | 7 (2.2%) | 33 (10.6%) |
| Nomikos et al. [226] | Amplatz + DVIU + ISD (1 yr.) | 34 | 12 | 8 (23.5%) | Stricture recurrence on urethroscopy/urethrography | 2 (5.8%) | NR | NR | 3 (8.8%) |
| Yu et al. [221] | Balloon | 31 | 15 (5-36) | 20 (64.5%) | Need for subsequent urethroplasty | 2 (6.5%) | 0 (0%) | NR | 1 (3.2%) |
DVIU = direct vision internal urethrotomy; FU = follow-up; ISD = intermittent self-dilatation; mo = months; N = number of patients; NA = not applicable; NR = not reported; UTI = urinary tract infection; yr = year.
6.2.2.2. Effectiveness of dilatation compared with direct vision internal urethrotomy
A SR identified only one prospective RCT comparing dilatation with DVIU and failed to detect any differences [196,220]. In a small (n=56) retrospective cohort study, the three-year estimated stricture recurrence-free survival was 35.5% and 28% for respectively balloon dilatation and DVIU (p=0.21) [221].
At present, there is lack of evidence to support the claim that dilatation is superior to DVIU (or vice versa) and therefore, the indications for single dilatation are the same as for DVIU.
Repetitive dilatation/DVIU with curative intent (see also section 6.2.1.1.3.6 Previous interventions) should be avoided as no long-term freedom of recurrence can be expected [223] and because of the significant risk of increasing stricture length and complexity and prolonging the time to urethroplasty (which has better patency rates) [222].
| Summary of evidence | LE |
| Visually controlled dilatation after endoscopic or fluoroscopic guidewire placement has a low complication rate. | 3 |
| Repetitive dilatations/direct vision internal urethrotomy have no long-term freedom of recurrence and increase stricture complexity. | 1b |
| Recommendations | Strength rating |
| Use visually controlled dilatation in preference to blind dilatation. | Weak |
| Do not perform repetitive (> 2) direct vision internal urethrotomy/dilatations if urethroplasty is a viable option. | Strong |
6.2.3. Post-dilatation/direct vision internal urethrotomy strategies
Several strategies have been developed and evaluated to prevent wound contraction, improve the stricture-free rate and time to stricture recurrence after dilatation or DVIU.
It is noteworthy that these strategies tend to stabilise the stricture rather than to keep the patient stricture-free and the reported outcomes should be understood in this respect.
6.2.3.1. Intermittent self-dilatation
6.2.3.1.1. Results
A SR identified six randomised and quasi-randomised trials comparing intermittent self-dilatation (ISD) with no ISD with a follow-up between eight and 24 months [227]. Stricture recurrence was reduced in men performing ISD (85/197, 43%) vs. those who did not (128/207, 62%) (RR: 0.70; 95% CI: 0.48-1.00; p=0.05). There was significant heterogeneity, and the quality of included studies were very low, which led the authors to conclude there is uncertainty about the estimate [227]. This review found no significant difference in adverse events between ISD and no ISD (RR: 0.60; 95% CI: 0.11-3.26; p=0.56) [227]. One trial containing 48 patients found no significant difference in six vs. twelve months duration of ISD (RR: 0.67; 95% CI: 0.12-3.64) and another trial (n=59) found no significant difference from using a low-friction hydrophylic vs. a polyvinyl chloride catheter (RR: 0.32; 95% CI: 0.07-1.40) [227]. Other studies have been published after this SR of 2014. Chhabra et al., reported that patients complying with ISD after dilatation had a lower need for re-intervention than those who did not, 12.3% vs. 20.5% respectively (p=0.2) [228]. After a mean follow-up of 25 months, Greenwell et al., found a need for subsequent intervention in 13/31 (42%) men performing ISD vs. 47/95 (49%) who did not (p=0.46). The number of re-operations in patients with need for subsequent intervention was lower in the group performing ISD vs. those who did not (2.6 vs. 3.4). No major complications were reported in both groups [229].
6.2.3.1.2. Complications
The potential benefit of ISD in stabilising the stricture must be balanced against the drawbacks. Commonly reported complications are urethral bleeding (7.1%) [230] and UTI/epididymitis (4.7-18.1%) [231,232]. A multicentric prospective study (n=85) reported that respectively 35% and 26% of patients had moderate to severe difficulties in catheterisation and respectively 32% and 17% of patients suffered moderate to severe pain while performing ISD. This had a serious impact on QoL which was rated moderate and poor in 32% and 55% of patients, respectively [33]. Younger age was identified as predictor for poor QoL, and QoL was more impaired in proximal stricture location (posterior and bulbar) [33]. In a study of 286 patients (mainly > 60 years old) performing ISD, 20% experienced problems with ISD and 33% had at least one infection annually. After a mean follow-up of 58 months 67% still continued with ISD [233]. Khan et al., reported eight “drop-outs” of 30 (26.7%) men randomised to ISD [232]. Of these eight “drop-outs”, two were unable to perform ISD and one stopped because of pain.
As mentioned above, repetitive dilatation (including ISD) increases stricture complexity and delays time to urethroplasty [222,234].
6.2.3.1.3. Intermittent self-dilatation combined with intra-urethral corticosteroids
To delay wound contraction at the stricture site, intra-urethral corticosteroids (as a catheter lubricant) have been used to improve the results of ISD. In 2014, a SR identified three prospective RCTs comparing ISD and local steroid (triamcinolone) ointment vs. ISD without local steroid ointment [235]. These three studies included a total 67 and 68 patients randomised to local steroid, or not, with a follow-up ranging between twelve and 36 months. There were fifteen (22.4%) recurrences in the steroid group and 25 (36.7%) in the control group (OR: 0.51; 95% CI: 0.24-1.10; p=0.09) [235]. Time to recurrence was longer in the steroid group vs. the control group (weighted mean difference = 0.29; 95% CI: 0.08-1.00; p=0.05). There was no difference in adverse events between groups [235].
Since 2014, two additional RCTs have been published. Ergun et al., evaluated patients after DVIU for primary short (< 2 cm), bulbar (82%) or posterior (18%) strictures that were further randomised between ISD (n=30) and ISD + triamcinolone ointment (n=30) for six weeks. Stricture recurrence rate after 24 months was not significantly different between ISD and ISD + triamcinolone (respectively 33.3 and 30%) [236]. On the other hand, Regmi et al., found a lower stricture recurrence rate (22% vs. 46%, p=0.04) in patients performing ISD + triamcinolone (n=27) vs. ISD alone (n=28) [237]. In this study, median time to recurrence was 7.4 ± 4.5 months vs. 11.9 ± 3 months in, respectively, ISD alone and ISD + triamcinolone (p=0.16). Both studies reported no complications related to triamcinolone ointment [236,237].
In a small (n=28) cohort with LS-related strictures, an intra-urethral steroid regimen was successful (no need for subsequent escalation of therapy) in 25 (89%) patients after a mean follow-up of 25 months [157]. This regimen consisted of applying clobetasol cream 0.05% as lubricant on a calibration device (10-16 Fr catheter or dilator) twice a day during a minimum of two months. As most of these patients further continued with instillation of steroids on a calibration device, this high “success” rate must be viewed with caution and should be considered as a stabilisation of the stricture rather than a cure. Eventually, twelve (42.8%) patients could reduce the interval of instillation/dilatation and three (10.7%) of them could finally stop the treatment [157].
| Summary of evidence | LE |
| Stricture recurrence was reduced in men performing intermittent self-dilatation (ISD) versus those who did not. | 1a |
| Intra-urethral corticosteroids in addition to ISD delays the time to recurrence. | 1a |
| Recommendations | Strength rating |
| Perform intermittent self-dilatation (ISD) to stabilise the stricture after dilatation/direct vision internal urethrotomy if urethroplasty is not a viable option. | Weak |
| Use intra-urethral corticosteroids in addition to ISD to stabilise the urethral stricture. | Weak |
6.2.3.2. Intralesional injections
The rationale of adjuvant intralesional injections is to reduce fibroblast proliferation and excessive urethral scaring [238].
6.2.3.2.1. Steroids
A 2014 SR identified five studies comparing intra-urethral submucosal steroid injection vs. no intra-urethral submucosal steroid injection after DVIU, of which two were RCTs [235]. Meta-analysis of these two RCTs with 57 and 58 patients in, respectively, the steroid and control group showed no statistical difference in recurrence rate (OR: 0.53; 95% CI: 0.25-1.13; p=0.10). Time to recurrence was significantly longer in the steroid group (weighted mean difference = 4.43; 95% CI: 2.77–6.09, p < 0.00001). There were no significant differences regarding adverse events (infection, bleeding, extravasation) between both groups (weighted mean difference = 1.59; 95% CI: 0.71–3.58, p=0.26).
6.2.3.2.2. Mitomycin C
In 2021 a SR and meta-analysis of different adjuncts to minimally invasive treatment of urethral stricture in men, mitomycin C (MMC) was associated with the lowest rate of urethral stricture recurrence (intralesional injection: OR: 0.23, 95% CI: 0.11-0.48; p=<0.001; intraluminal injection: OR: 0.11, 95% CI: 0.02-0.61; p=0.01) [239]. Another SR and meta-analysis in 2021 from Xu et al., on the efficacy of MMC combined with direct vision internal urethrotomy, revealed that the effect of MMC was significant in short (< 2cm), anterior urethral strictures, in the longer (>12 months) follow-up group [240].
In the absence of well-conducted and adequately powered RCTs along with the lack of standardisation (dose, technique, volume, etc.) in the current literature, careful clinical review and prospective data collection as part of a clinical trial is advised.
6.2.3.2.3. Platelet rich plasma
Rezaei et al., conducted an RCT comparing DVIU + platelet rich plasma (PRP) (n=44) vs. DVIU + saline (n=43) in primary, bulbar strictures < 1.5 cm in length [241]. The two-year stricture-free rate was 78% vs. 56% after DVIU with or without PRP, respectively (p=0.034). Complications were frequent but not significantly different between both groups (DVIU + PRP: 70%; DVIU + saline: 79%). All complications (urethral bleeding, haematuria, urethral pain, pelvic pain, urinary leakage and genitoperineal swelling) were classified as grade 1 according to the Clavien-Dindo system. Further validation of this treatment is needed before general clinical implementation.
| Summary of evidence | LE |
| Intralesional injections after direct vision internal urethrotomy (DVIU) might improve stricture-free rates on the short-term compared to DVIU alone. Experience is limited and the use of these drugs are off-label. Significant uncertainty exists about drug, dose, volume and technique. | 1a |
| Recommendation | Strength rating |
| Use intralesional injections only in the confines of a clinical trial. | Weak |
6.2.3.3. Urethral stents
Urethral stents are designed with the aim to oppose wound contraction after dilatation or DVIU [242,243]. Stent insertion is a short procedure (< 60 minutes) that can be done under local or spinal anaesthesia as a “one-day” surgery [242,244,245]. Urethral stents are classified as permanent or temporary (removable, after six to twelve months).
6.2.3.3.1. Results
Permanent stainless-steel mesh stents are no longer commercially available. An RCT comparing dilatation/DVIU only vs. dilatation/DVIU followed by temporary stent insertion for bulbar strictures reported a significantly longer stricture-free survival time in favour of dilation/DVIU followed by stent (median 292 vs. 84 days; p < 0.001) [246]. Only 20.6% of patients treated with a stent developed a recurrent stricture within one year vs. 82.8% in the control group. These results are corroborated by a prospective series of Wong et al., who found a median stricture-free survival of two months after DVIU alone vs. 23 months after DVIU followed by temporary (three months) stent for bulbar strictures [243].
Failure and need for re-intervention are frequent (30-53%) and are usually because of stricture recurrence, stent encrustation, stent migration and urethral hyperplasia. Other complications include recurrent UTI, recurrent haematuria and genito-perineal pain (Table 6.5). Although stents are mainly used to treat bulbar strictures, they have been used for posterior stenoses as well. Stents used in the posterior urethra have a high risk (82-100%) of causing UI and this is most pronounced in patients with previous irradiation and/or strictures extending into the membranous or bulbar urethra [247]. In the bulbar urethra, the risk of UI is higher if stent placement is adjacent to the external sphincter [248]. The use of stents in the penile urethra is anecdotal. Jung et al., reported stent failure in 4/7 (57%) patients with a penile stricture after a mean follow-up of eight months. Of those patients who failed, no patient with distal or pan-penile strictures was rendered stricture-free [249]. In their series, stricture recurrence after stenting of the penile urethra was significantly higher when compared to the bulbar urethra [249]. Although no direct comparison is available, temporary stents tend to have fewer and less severe complications compared to permanent stents (Table 6.7).
6.2.3.4. Drug-coated balloon dilatation
Drug (paclitaxel)-coated balloon dilatation (DCBD) after standard dilatation or DVIU aims to reduce scar formation based on its antimitotic action. The ROBUST-3 trial prospectively randomised patients with predominantly bulbar strictures (< 3cm length) and at least two prior failed endoscopic treatments to DCBD (n=79) or standard dilatation/DVIU (n=48) [250]. Anatomic patency (assessed by cystoscopy) at six months was 75% for DCBD versus 27% after standard dilatation/DVIU (p <0.001). Estimated one year-retreatment free survival was 83% versus 22% for respectively DCBD and standard dilatation/DVIU (p <0.001). There were no serious adverse events related to DCBD although patients undergoing DCBD had a higher rate of hematuria and dysuria compared to controls (11.4% versus 2.1%). Paclitaxel was detected in semen up to six months after treatment which urges for contraception if the partner has child-bearing potential. If used, careful clinical review and prospective data collection ideally part of a post marketing registry or clinical trial is advised.
| Summary of evidence | LE |
| Drug (paclitaxel)-coated balloon dilatation is associated with higher anatomic patency rates (at six months) and lower risk of retreatment (at one year) as compared to standard dilatation/DVIU in patients with short (< 3 cm), bulbar strictures that underwent at least two prior failed endoscopic treatments. | 1b |
| Recommendation | Strength rating |
| Offer drug (paclitaxel)-coated balloon dilatation for a short (< 3 cm) bulbar stricture recurring after at least two prior endoscopic treatments, but only in patients for whom urethroplasty is not an option. | Weak |
6.2.3.4.1. Treatment of stent failure
In the case of stent failure, subsequent urethroplasty (usually with stent removal) is possible, but this urethroplasty is very likely to be more complex than it would have been had it been performed initially [251-253]. Due to the fact that the stainless-steel wires are fully embedded into the urethral wall, over time the urethral spongiosum is severely damaged. Horiguchi et al., found that a history of urethral stenting was an independent significant predictor of increased stricture complexity (OR: 13.7; 95% CI: 1.7-318.3; p=0.01) and need for more complex urethroplasty (OR: 6.9; 95% CI: 1.1-64.5; p=0.04) [234]. The majority (62%) of patients in this study had a permanent stent which tend to be difficult to remove because they are epithelialised, usually within six months [234]. The type of urethroplasty required depends on the length of the stricture and quality of local tissues [252]. In the majority of cases, it is possible to preserve the urethral plate and to perform a one-stage substitution urethroplasty [251,252,254]. The patency rates after different types of urethroplasty vary greatly between 16.7-100% [251-254] and this variation probably reflects variation in complexity of the stricture, rather than that the superiority of one technique of urethroplasty over another (for further information see supplementary Table S6.2). Due to these limitations, the use of stents should be avoided if subsequent urethroplasty is considered [242,253]. Urethral stents are not a first-line treatment for urethral strictures but can be considered in co-morbid patients who have a recurrent stricture after DVIU/dilatation and are unable to have more complex urethroplasty or who refuse urethroplasty [242,246,247].
| Summary of evidence | LE |
| Permanent urethral stents have a high complications and failure rate and make subsequent urethroplasty more challenging if they fail. | 3 |
| Stents have a higher failure rate in the penile urethra. | 3 |
| Temporary stents after DVIU (direct vision internal urethrotomy)/dilatation at the bulbar urethra prolong time to next recurrence compared to DVIU/dilatation alone. | 1b |
| Recommendations | Strength rating |
| Do not use permanent urethral stents. | Strong |
| Do not use urethral stents for penile strictures. | Strong |
| Use a temporary stent for recurrent bulbar strictures after direct vision internal urethrotomy to prolong time to next recurrence only if urethroplasty is not a viable option. | Weak |
Table 6.5: Failure rate and complications associated with urethral stents

DVIU = direct vision internal urethrotomy; FU = follow-up; NR = not reported; UI = urinary incontinence; UTI = urinary tract infection; VUAS = vesico-urethral anastomotic stricture; Qmax= maximum flow rate.
6.3. Open repairs (urethroplasty): site and aetiology (clinical scenario) treatment options
6.3.1. The role of urethroplasty in the management of penile urethral strictures
Due to the specific aetiology and the associated problems, strictures related to failed hypospadias repair and LS will be discussed separately. However, many series reporting on the outcome of penile strictures have a mixed aetiology also including failed hypospadias repair and/or LS [255,256]. Due to their specific location, distal penile strictures will be discussed separately.
6.3.1.1. Staged augmentation urethroplasty
Classically called “two-stage” urethroplasty, this approach may become a multi-stage urethroplasty as revision (usually due to graft contracture) after the first stage has been reported in 0-20% of cases [256-259]. Therefore, the term “staged” should be used instead [260]. Revision rates before second stage were 0-20%, stressing that a two-stage urethroplasty might become a multi-stage urethroplasty. In general, reconstructive urologists tend to follow this approach in men with more complex urethral stricture disease (multiple interventions in the past, unfavourable clinical findings such as significant spongiofibrosis or scarring that requires excision, poor quality of the urethral plate). An interval of at least four to six months has been proposed before proceeding to the tubularisation of the urethra, provided that the graft has healed uneventfully [261-263].
A SR by Mangera et al., has shown an average patency rate of 90.5% with the use of all types of grafts for staged penile urethroplasties with an average follow-up of 22.2 months [264]. Patency rates of staged oral mucosa graft (OMG) urethroplasty in specific locations vary between 73.3 and 100% [255,256,258,259,265,266]. Post-operative urethrocutaneous fistula (UCF) rates were 17.2% and 2.6% in the studies of Ekerhult et al., and Joshi et al., respectively, and either not reported or unclear in the remaining studies [255,256].
6.3.1.2. Single-stage augmentation urethroplasty
Single-stage urethroplasty offers the option for reconstruction of the stricture without the need for multiple operations, the associated peri-procedural risks, and the cosmetic and functional implications that by definition follow the first part of staged urethroplasties [267-269]. There is some evidence to suggest a considerable number of patients (50% or more in some studies) who were offered first stage urethroplasty never returned for the second stage because they were either satisfied with their functional status after the first stage (this particularly applied to older men or patients with multiple failed procedures in the past) or they were disappointed with the need for another operation [267,268].
In the SR of Mangera et al., overall patency rate for all types of single-staged graft urethroplasties is 75.7% with an average follow-up of 32.8 months [264].
The patency rate for different one-stage techniques in particular are:
- dorsal OMG (n=320): 63-100% [265,266,259,270-276];
- ventral OMG (n=54): 55-92.6% [265,277,278];
- double (dorsal + ventral) onlay with penile/scrotal skin graft /OMG (n=14/8/4): 88.5% [272];
- dorsal penile skin graft (n=44): 62-78% [272,273];
- penile skin flap (n=367): 67-100% [265,266,272-274,279].
No high-level evidence exists to state that one technique is superior to another, but it seems that the dorsal graft location is more commonly used compared to the ventral one. Mangera et al., reported that the patency rate was better with OMG compared to other grafts (mainly penile skin) [264]. Jiang et al., showed that combined (dorsal + ventral) BMG onlay had significantly better stricture-free rates for penoscrotal strictures (patency rate 88.9% vs. 60.9% with single-onlay approach); however, follow-up was significantly shorter in the double-onlay group [280]. Few studies have reported dedicated results on sexual function parameters that do not appear to be significantly impaired post-operatively [258,281].
A critical factor with respect to single-staged procedures is the careful selection of patients, as men with long and complex strictures might not be good candidates for single-stage reconstruction and attempts to offer single-staged operations in these patients might lead to higher recurrence rates. Sometimes, this selection can only be done based on intra-operative findings. Therefore, any scheduled single-staged procedure might be converted into a staged one [267,282]. Palminteri et al., highlighted the fact that single-stage augmentation urethroplasties in men with LS-related strictures enlarge rather than remove the diseased segment of the urethra; therefore, there is always a risk of recurrence in the future [283]. The role of previous interventions (especially multiple urethrotomies or history of previous urethroplasties) remains unclear as several studies on single-staged operations do not provide information on previous procedures, or excluded patients with operations in the past [274,281]. Although favourable outcomes in patients with previous history of urethrotomies/urethroplasties were reported by Barbagli and Kulkarni, in the study by Pfalzgraf et al., all recurrences post-previous urethroplasty took place in the single-stage group while Ekerhult et al., identified prior history of urethral operations as a risk factor for recurrence in the group of single-stage procedures [255,258,259,272]. In addition to previous urethral surgery, high BMI has also been identified as a poor prognostic factor after single-stage penile urethroplasty [255].
6.3.1.3. Anastomotic urethroplasty in men with penile urethral strictures
Historically, the use of anastomotic urethroplasty in the management of urethral stricture disease has been discouraged due to the risk of chordee post-operatively [263,284]. Nevertheless, it has been performed in selected patients with very short strictures (usually < 1 cm) with 80-93% patency rate, with satisfactory QoL and sexual function and without any case of chordee [285], and with results comparable to augmentation urethroplasty [286].
| Summary of evidence | LE |
| Stricture-free rates for single-stage penile augmentation urethroplasties range from 70-100% for dorsal OMG augmentation, 67-100% for penile skin flap (PSF) augmentation, 55-92.6% for ventral OMG augmentation and 62-78% for dorsal SG augmentation. Overall stricture-free rates for staged OMG penile augmentation urethroplasties range from 70-100%. | 2b |
| In staged urethroplasties, an interval of at least four to six months has been proposed before proceeding to the tubularisation of the urethra, provided that the graft has healed uneventfully. | 4 |
| The use of anastomotic urethroplasty in the management of urethral stricture disease has been discouraged due to the risk of chordee post-operatively. Anastomotic urethroplasty can be offered in selected cases of very short (< 1 cm), injury-associated penile strictures. | 3 |
| In case of adverse intra-operative findings, a single-stage approach might not be feasible and must be converted into a staged approach. | 3 |
| Recommendations | Strength rating |
| Offer men with penile urethral stricture disease augmentation urethroplasty by either a single-stage or staged approach taking into consideration previous interventions and stricture characteristics. | Strong |
| Offer an interval of at least four to six months before proceeding to the second stage of the procedure provided that outcome of the first stage is satisfactory. | Weak |
| Do not offer anastomotic urethroplasty to patients with penile strictures > 1 cm due to the risk of penile chordee post-operatively. | Strong |
| Counsel patients with penile strictures that single-stage procedures might be converted to staged ones in the face of adverse intra-operative findings. | Strong |
6.3.1.4. Specific considerations for failed hypospadias repair-related strictures
The term “failed hypospadias repair” (FHR) includes a wide range of abnormalities after previous attempts for reconstruction, such as glans deformity, recurrent urethral stricture, glans/urethral dehiscence, UCF and penile chordee [287-289]. The management of FHR is challenging as the urethral plate, penile skin and dartos fascia are often deficient/non-existent. Management of these patients is often made more difficult due to incomplete health records and a lack of critical information (original meatal site, number, and type of previous repairs) [261,290]. In addition, multiple operations might need to be offered to reach satisfactory outcomes [287]. As a result, FHR should always be considered as a complex condition and it is advised that FHR management takes place in high-volume centres [288,289,291,292].
“Hypospadias cripples” is a term widely used to describe the group of men with multiple previous failed attempts to correct the condition resulting in unfavourable results such as severe scarring, penile deformity and shortening, hair or stones in the urethra, UCF, chordee and functional disorders (e.g., urinary, or sexual dysfunction). This term should be avoided and a more neutral one should replace it as it further stigmatises men with hypospadias who have been shown to have reduced self-esteem and confidence due to unsatisfactory cosmesis, and problematic urinary and sexual function. Moreover, it has been reported that FHR patients experience high rates of disappointment after failure of attempted repair and a sense of helplessness as they are frequently advised that their failed hypospadias is too complex to correct and they should not pursue further repair [288-290,293,294].
Two main approaches are applicable: single-stage or staged procedures. In general, it is advised that staged procedures should be followed when the urethral plate is inadequate for a single-stage operation. Surgeons should gain the consent of patients for both types of urethroplasty as the surgical approach might need to be modified intra-operatively, depending on favourable/unfavourable intra-operative findings. Besides poor-quality of the urethral plate, these unfavourable findings include high degree of scarring and presence of concomitant LS, UCF and/or chordee. It is not uncommon for men with FHR to have scarred skin or concurrent LS; thus, skin grafts or flaps should be avoided as the risk of recurrence due to LS is very high (90% in long-term follow-up as reported by Depasquale et al.) [39,295,296].
Staged repairs (using mainly BMG) reported patency rates ranging from 71-95% [293,295,257,297,298], while single-stage repairs had patency rates from 80-100% [295,297,299-302]. It needs to be highlighted that, as FHR is an umbrella term that covers various clinical conditions apart from urethral stricture disease only (such as UCF, chordee, penile deformity), “success” rates as reported by the authors in their studies do not represent urethral patency rates only. Unfortunately, the number of previous operations is either not reported or refers to the whole FHR study group collectively rather than to the subgroups of staged/single-staged procedures.
A comparative analysis is reported by Barbagli et al., in 345 FHR patients at five-year follow-up. Overall failure-free survival rate was 48% for all urethroplasties, and in sub-analysis, staged techniques had significantly lower treatment failure-free survival rates compared to single-stage techniques [303]. However, it is unclear whether these groups were comparable in terms of baseline characteristics such as age, length of stricture, number of procedures, comorbidities etc. [303]. If the patients in the staged group had a more unfavourable background, this on its own could explain the final outcome rather than the surgical approach itself.
Kozinn et al., reported a 16% and 14% revision rate after the first and second stage, respectively, and observed that these revision rates were higher in the FHR group compared to non-FHR patients with penile strictures [257]. There is conflicting evidence whether FHR as aetiology is a poor prognostic factor in the outcome of urethroplasty for penile strictures [255,304-306]. Concomitant UCF can be successfully managed at the same time of urethroplasty [303].
Saavendra et al., reported 89.3% stricture-free rate in 56 FHR patients with penile urethral strictures at a median of 21 months follow-up using mainly staged urethroplasty and perineal urethrostomy. Verla et al., presented departmental experience with the use of various urethroplasty techniques in a total of 76 FHR patients with penile strictures. Follow-up was long, at a median of 89 months and stricture-free rates ranged from 29% (anastomotic repair) to 90% first stage only of multistage urethroplasty).
For further information see supplementary Table S6.3.
| Summary of evidence | LE |
| Men with failed hypospadias repair (FHR) have history of multiple interventions, and poor-quality tissues, and might require complex procedures for a satisfactory functional and cosmetic outcome. | 4 |
| Men with FHR may have low self-esteem due to urinary and sexual dysfunction and unsatisfactory cosmesis. | 2b |
| Men with FHR can have scarred penile skin or concurrent lichen sclerosus and outcomes with skin grafts or flaps can be unsatisfactory. | 3 |
| Recommendations | Strength rating |
| Men with failed hypospadias repair (FHR) should be considered complex patients and referred to specialist centres for further management. | Weak |
| Propose psychological and/or psychosexual counselling to men with unsatisfactory cosmesis and sexual or urinary dysfunction related to FHR. | Weak |
| Do not use penile skin grafts or flaps in failed FHR patients with lichen sclerosus or scarred skin. | Strong |
6.3.1.5. Specific considerations for lichen sclerosus-related penile urethral strictures
Given the fact that LS affects the skin, the use of genital skin as a flap or graft is not advised as the risk of disease recurrence has been reported to be high (50-100%) and while most recurrences tend to occur within the first two to three post-operative years, late recurrences have been reported [307].
Main strategies are single-stage or staged oral mucosa graft urethroplasty.
The EAU Urethral Strictures Guidelines Panel conducted a SR [6] to explore the role of single-stage oral mucosa graft urethroplasty in the management of LS-related urethral strictures and to compare its outcomes with alternative management options (surgical dilatations +/- ISD; surgical dilatations + local steroids +/- ISD; staged oral mucosa urethroplasty; penile skin urethroplasty; meatotomy/meatoplasty; urethrotomy [Otis, DVIU]; perineal urethrostomy; urinary diversion [e.g., suprapubic catheterisation]).
In total, fifteen studies met the inclusion criteria, recruiting a total of 649 patients (366 from five non-randomised comparative studies and 283 from ten, single-arm retrospective observational studies). Single-stage OMG urethroplasty resulted in success rates ranging from 65-100% after twelve to 67 months mean or median follow-up. For staged OMG urethroplasty, the most commonly reported comparator, the success rates were somewhat lower and varied between 60-79%. Methodological issues (mainly selection bias) could explain the difference in success rates rather than the intervention itself. Complications were uncommon (0-12%) and mainly comprised Grade 1-3 events.
Due to the overall very poor quality of evidence, the SR did not provide a clear answer as to whether single-stage OMG urethroplasty is superior to other management options, although careful patient selection is highlighted. In the absence of adverse local tissue conditions, a single-stage approach could lead to high success rates with an improvement in voiding symptoms and QoL.
| Summary of evidence | LE |
| Lichen sclerosus is a skin condition that can lead to scarring, and recurrence rates after skin graft/flap augmentation urethroplasties have been reported to be high (50-100%). | 4 |
| Single-stage oral mucosa graft (OMG) urethroplasty provides patency rates between 65 and 100% and is not inferior to staged OMG urethroplasty. | 3 |
| Recommendations | Strength rating |
| Do not use genital skin in augmentation penile urethroplasty in men with lichen sclerosus (LS) related strictures. | Strong |
| Perform single-stage oral mucosa graft urethroplasty in the absence of adverse local conditions in men with LS related strictures. | Weak |
6.3.1.6. Distal urethral strictures (meatal stenosis, fossa navicularis strictures)
Open repair of distal urethral strictures can be in the form of Malone meatoplasty, skin flap meatoplasty or graft (skin [SG]/OMG) urethroplasty.
For short distal meatal strictures, the Malone meatoplasty (dorsal + ventral meatotomy) provides a technique with patency rates up to 100%, while 83% of patients reported satisfaction with the cosmetic results [308].
Skin flap meatoplasty showed excellent patency rates ranging from 90-96% based on three studies comprising 67 patients [309-311]. In addition, based on their results, patient satisfaction with post-operative outcomes and cosmesis was high, there were no cases of ED and functional complaints were minimal (mainly spraying of the urine flow).
Patency rates with the use of grafts (OMG or SG) ranged from 69-91% in 106 patients overall [312,313,300]. Where reported, patients were satisfied with cosmesis, and mild spraying of the urine flow self-resolved. Although tubularised grafts in a single-stage procedures are not routinely recommended (see also section 9. Tissue transfer), one series reported an 89.9% patency rate for this approach (“two-in one approach”) in selected patients with mainly distal penile strictures [314].
Finally, Daneshvar et al., presented a novel transurethral ventral inlay OMG technique and showed excellent stricture-free rates (96%) at short follow-up though (median 16 months).
For further information see supplementary Table S6.4.
| Summary of evidence | LE |
| Post-meatoplasty/urethroplasty patency rates in men with meatal stenosis or fossa navicularis/distal urethral strictures range between 57-100% depending on type of surgical intervention with high patient satisfaction and minimal complications. | 3 |
| Recommendation | Strength rating |
| Offer open meatoplasty or distal urethroplasty to patients with meatal stenosis or fossa navicularis/distal urethral strictures. | Weak |
6.3.2. Urethroplasty for bulbar strictures
6.3.2.1.“Short” bulbar strictures
The length of a “short” bulbar stricture is poorly defined. In general, “short bulbar strictures” are those amenable to stricture excision and subsequent tension-free anastomotic repair. The limit is usually around 2 cm [315].
In fit patients, the choice of urethroplasty is between EPA (transecting or non-transecting) and FGU.
6.3.2.1.1. Excision and primary anastomosis
6.3.2.1.1.1. Excision and primary anastomosis with transection of corpus spongiosum (transecting EPA)
Transecting EPA (tEPA) is based on the full thickness resection of the segment of the bulbar urethra where the stricture and surrounding spongiofibrosis is located. Reconstruction is performed by a tension-free spatulated anastomosis.
6.3.2.1.1.1.1.Patency rates
The International Consultation on Urological Diseases (ICUD) performed an extensive review of the literature and reported a composite patency rate of 93.8% for tEPA [316]. Based on this, they endorsed tEPA as treatment of choice for short bulbar strictures if other techniques have an expected patency rate below 90%. However, penile complications were not taken into account for this advice and as discussed below, these are a concern with tEPA.
Prospective data report a patency rate of 88% at twelve months follow-up [315].
Usually, no need for further intervention is used to evidence that the urethra is patent. In the few studies using an anatomic definition for failure (an inability to pass a 16 Fr endoscope) tEPA urethroplasty achieves a similar patency rate, ranging between 85.5-97% [145,317-319] (Table 6.12). The median time for recurrence after tEPA is between 3.5 and thirteen months [145,320,321].
Several authors suggested that tEPA is the technique of choice for short post-traumatic bulbar strictures with complete obliteration of the urethral lumen and full thickness spongiofibrosis [319,322]. These strictures are a specific entity and usually the result of a straddle injury with complete or nearly complete rupture of the bulbar urethra. These obliterations are predominantly short and can be treated with tEPA yielding a patency rate of 98.5% as reported in the series of Horiguchi et al., [323]. They also reported an improvement in erectile function after urethroplasty measured one year post-operatively. Straddle injury (and perineal trauma) are a common aetiology in papers published about tEPA; however, separate data on the outcomes for this specific aetiology is usually lacking.
6.3.2.1.1.1.2.Complications
Nilsen et al., conducted an RCT comparing tEPA with FGU for short (< 2 cm) bulbar strictures [315]. Compared to FGU, penile complications were more frequent with tEPA. After three months, worse ejaculation (26%), reduced glans filling (26%), penile shortening (16%) and penile chordee (10%) were significantly more reported with tEPA. After twelve months, reduced glans filling (19%) and penile shortening (26%) remained significantly more reported with tEPA. A scrotoperineal hematoma was significantly more frequent with tEPA compared to FGU (resp. 24 versus 4%). Despite these complications IIEF-5 was not significantly different between both groups at three and twelve months.
These latter complications (as well as ED) might be attributed to complete transection of the corpus spongiosum at the level of the stricture, thereby disrupting the antegrade blood flow of the urethra and corpus spongiosum. To spare this, the non-transecting EPA (ntEPA) has been described [324] and later modified [325].
6.3.2.1.1.2. Non-transecting excision and primary anastomosis
6.3.2.1.1.2.1.Patency rates
Except for straddle injuries that are usually associated with complete obliteration of the lumen and full thickness scarring of the corpus spongiosum [319,326], ntEPA is a good alternative for short bulbar strictures of all other aetiologies. With median follow-ups ranging between 17.6 and 37.1 months, the patency rates reported are 93.2-99%; with the lack of further intervention as success criteria [322,327,328]. Even with the anatomic criteria (16 Fr cystoscopy passage) the success rate achieved was 97.9% at twelve months [319] (see supplementary Table S6.7).
Two comparative analyses evaluated tEPA vs. ntEPA. Waterloos et al., reported patency rates of 88.4% and 93.2%, respectively, for tEPA and ntEPA (p=0.33) but with significantly longer follow-up for tEPA (118 vs. 32 months, p < 0.001). Of patients scheduled for ntEPA, 11.1% were converted to tEPA, highlighting that ntEPA is not always possible. Chapman et al., using anatomic success criteria (16 Fr cystoscope passage), reported patency in 93.8% of tEPA vs. 97.9% of ntEPA. Follow-up was also significantly shorter at 74.1 (SD: 45.4) months for tEPA vs. 37.1 (SD: 20.5) months for ntEPA (p < 0.001) [319].
6.3.2.1.1.2.2.Complications
When erectile function after urethroplasty was assessed (at six months), ntEPA had significantly lower ED rates (a decrease of > 5 points on the sexual health inventory for men [SHIM] scale) compared to tEPA (4.3 vs. 14.3%, respectively) [319]. Urethral transection performed during tEPA was the only factor associated with sexual dysfunction in a multivariate analysis [319]. Other series reported ED lasting for more than six months in 2-6% of cases after ntEPA [322,328,329]. Grade > 2 Clavian-Dindo complications were 3.6-8.1% vs. 4.3-6.8%, respectively, for tEPA and ntEPA, without reaching statistical significance [319,327].
To date, no trials comparing ntEPA with FGU have been published to report on comparative patency outcomes and complications.
6.3.2.1.2. Free graft urethroplasty
Despite the very high patency rates of EPA, FGU has been performed for short bulbar strictures as well. This is mainly driven by reports of ED after EPA. A meta-analysis of ten papers [340] comparing tEPA with BMG FGU for short strictures, found that tEPA is better than BMG FGU in terms of patency rates (91.5% vs. 70%), whilst BMG FGU has less erectile complications (9% vs. 25%). However, the methodology of this meta-analysis must be disputed as it was performed on cohort studies without risk of bias assessment and without further specification of timing of assessment of ED. On the other hand an RCT comparing tEPA with BMG FGU, found no significantly different patency rates for EPA compared to BMG FGU (88% vs. 87% respectively) and no significant differences in erectile function for tEPA compared to BMG FGU [315]. As mentioned earlier, penile complications were more frequent with EPA.
Dogra et al., [283] looked prospectively at sexual function in 87 patients after different urethroplasties (EPA, penile/bulbar substitution) and found a 20% reduction in sexual function in all groups, which resolved after six months.
Details on where to place the graft during FGU are discussed below.
| Summary of evidence | LE |
| For short post-traumatic strictures tEPA has good patency rates. | 3 |
| For short bulbar strictures not related to straddle injury tEPA, ntEPA and FGU have the same patency rates, but ntEPA and FGU have less erectile dysfunction or penile complications than tEPA. | 1b-3* |
*LE1b for comparison between tEPA and FGU and LE3 for tEPA versus ntEPA versus FGU.
| Recommendations | Strength rating |
| Use transecting excision and primary anastomosis (tEPA) for short post-traumatic bulbar strictures with (nearly) complete obliteration of the lumen and full thickness spongiofibrosis. | Strong |
| Use non-transecting excision and primary anastomosis or free graft urethroplasty instead of tEPA for short bulbar strictures not related to straddle injury. | Weak |
6.3.2.2.“Longer” bulbar strictures
6.3.2.2.1. Free graft urethroplasty
For strictures not amenable to EPA, FGU is the technique of choice and buccal mucosa is, at the moment, the most widely used graft. Other grafts (and flaps) are possible and discussed in the tissue transfer chapter. Patency rates of FGU of the bulbar urethra are 88-91% with twelve to 40 months follow-up [264,330]. There is a suggestion that patency rates deteriorate with time [331].
During bulbar urethroplasty, the bulbospongiosus muscle is usually separated at the midline which may cause damage to the muscle and perineal nerves. This might subsequently provoke post-void dribbling and ejaculation disorders. In order to reduce this, the muscle and nerve-sparing perineal approach has been introduced [332]. Although it is mostly used in graft urethroplasty, this approach is also possible for EPA [333]. Elkady et al., [329] randomised 50 patients between a muscle and nerve-sparing perineal approach vs. a classic perineal approach and found no difference in operative time (100 vs. 105 min), but significantly less dribbling (4% vs. 36%, p=0.01), and significantly less ejaculatory changes (8% vs. 40%, p=0.02) in the nerve and muscle-sparing group. Fredrick et al., [333] did the same in 50 patients in a multicentric study with bulbar urethroplasty but could not find a statistical difference regarding post-void dribbling and ejaculatory changes. Due to the limited and conflicting evidence, no recommendation can be made about the routine use of nerve and muscle-sparing modification during bulbar urethroplasty.
See supplementary Table S6.8 for further information.
6.3.2.2.2. Augmented anastomotic repair
Augmented anastomotic repair has been described for these strictures. It has been mainly performed in cases where the stricture was just too long (+/- 2-4 cm) for tension-free EPA [334]. It can also be performed for longer strictures with a shorter (nearly) obliterative segment [335]. In this case, only the most obliterative segment is excised, the urethral plate is anastomosed, and the urethra is further reconstructed with an onlay graft [335]. Patency rates after AAR vary between 91.1-91.9% with twelve to 28 months follow-up [334]. The use of this technique has been challenged by Redmond et al., who found a 4.8 higher risk of recurrence when AAR was used compared to (dorsal) free graft urethroplasty [336] (see supplementary Table S6.9).
A non-transecting alternative has also been described to overcome the previously mentioned inconveniences related to spongiosal transection (augmented non-transecting anastomotic bulbar urethroplasty [ANTABU]). With this technique, Bugeja et al., [337] reported a 100% patency rate in sixteen patients after a median follow-up of thirteen months. One patient (6.7%) suffered permanent ED.
| Summary of evidence | LE |
| For strictures not amenable to EPA, FGU provides an 88-91% patency rate at short to medium follow-up. | 1b |
| Augmented anastomotic repair provides good (88-92%) patency rates for bulbar strictures with a nearly obliterative segment, despite deterioration with time. | 3 |
| Recommendations | Strength rating |
| Use free graft urethroplasty for bulbar strictures not amendable to excision and primary anastomosis (EPA). | Strong |
| Use augmented anastomotic repair for bulbar strictures not amenable to EPA but with a short, nearly obliterative segment within the whole strictured segment. | Weak |
6.3.2.2.3. Location of the graft during urethroplasty for bulbar strictures
The best location for graft positioning into the bulbar urethra remains to be determined. There are many techniques described with ventral, lateral, dorsolateral, or dorsal graft as an onlay or an inlay. Onlay means from the outside onto the urethra, inlay means from the inside after opening the urethra.
Regarding the site of graft placement, the Panel has conducted a SR assessing the literature from 1996 onwards, including studies with at least 20 patients and a minimum of twelve months follow-up [7]. This yielded one RCT, four non-randomised comparative series and 36 case series comprising 3,683 patients. The RCT of Vasudeva et al., compared ventral (n=40) with dorsal (n=40) onlay BMG urethroplasty and reported a patency rate of 90-92.5%, respectively at twelve months follow-up (p=0.51) [330]. The non-randomised comparative studies could not identify any significant differences in patency rates for dorsal onlay vs. ventral onlay, dorsal inlay vs. ventral onlay, or dorsal onlay vs. ventral onlay vs. dorsolateral onlay. Case series reported a patency rate of 62.1-98.3% for dorsal onlay, 74.3-94.4% for ventral onlay and 78.4-92% for dorsal inlay. There are no arguments to assume a higher risk of ED with one of the four techniques. Post-void-dribbling was reported in 0-28.1% with dorsal onlay and in 20-21% with ventral onlay. Other complications were also similar in incidence between techniques. Urethrocutaneous fistula and urethral diverticulum were only reported with the ventral onlay technique although this consisted of only two and one cases, respectively.
Double ventral-dorsal onlay, proposed for high-grade/nearly obliterative strictures, yielded a patency rate of 90-91% after 22-33 months follow-up [146,338].
| Summary of evidence | LE |
| Location of the graft has no impact on patency rates. | 1b |
| Recommendation | Strength rating |
| Use dorsal, dorsal-lateral, or ventral approach according to surgical practice, expertise, and intra-operative findings. | Strong |
6.3.2.3. Staged urethroplasty for bulbar urethral strictures
6.3.2.3.1. Indications
Staged urethroplasty may be considered when:
- there are locally adverse conditions such as fistula, false passage, abscess, cancer [282,339,340];
- there has been a previously unsuccessful complex urethroplasty including failed hypospadias repair [257,339];
- there is a lack of certainty on behalf of the surgeon regarding the most appropriate form of urethroplasty for the patient [339];
- the stricture is radiotherapy induced [257];
- the stricture is consequent to LS [257] (this is controversial and for some groups LS is a contraindication for a staged urethroplasty [305]; Kozinn et al., recommend leaving at least ten months between 1st stage and 2nd stage re-tubularisation in patients with LS to allow graft complication to develop) [257];
- there is severe spongiofibrosis [341].
6.3.2.3.2. Outcomes
Patency rates of 33.3-94.6% at mean follow-up of 11.2-50 months have been described for staged urethroplasty in series which include men with bulbar urethral stricture disease [257,305,318,341-343]. Grafts (mesh graft, preputial skin, oral mucosa) can be used in staged augmentation as well as marsupialisation [318,341]. In patients affected by LS, a 52.2% patency rate for staged urethroplasty was reported whereas this was 86% for single-stage buccal mucosa urethroplasty (p < 0.01) [305]. It is highly likely that different stricture and patient characteristics contributed to the differences reported and this should be kept in mind when interpreting the data. Of note, 19-45.5% of patients planned for staged urethroplasty declined to proceed to 2nd stage re-tubularisation [257,342].
Early complications after staged procedures include wound dehiscence, UTI, epididymitis, scrotal abscess, and penile numbness. Specific to 2nd stage Johanson urethroplasty UCF occurs in 3-15%. The actual incidence of UCF is probably higher as many small fistulae close spontaneously with conservative management and are not formally reported [305,318,341].
Late complications of 1st stage urethroplasty include a need for revision in up to 19% - as a consequence of recurrence of LS in graft(s) (8.8%), graft contracture (6.6%) and stomal stenosis (3.3%) [257]. Late complications of 2nd stage urethroplasty include post-micturition dribble in 14-18%, SUI in up to 16%, penile curvature in up to 9%, ED in up to 4%, urethral diverticulum formation in 1% and cold glans [305,341,343]. Stress urinary incontinence, penile curvature and ED appear to be particularly associated with mesh graft stage urethroplasty [341,343].
After their procedure, 86% and 96.6% of men with, respectively, mesh graft and buccal mucosa graft staged urethroplasty were satisfied. The patient groups included in the review were too small to detect significant differences [341]. All are retrospective series – with heterogenous indications, stricture locations (not exclusively bulbar), stricture lengths and patient groups. It is consequently difficult to draw meaningful conclusions from the little data that are available.
See supplementary Table S6.10 for more information.
| Summary of evidence | LE |
| Staged urethroplasty for bulbar strictures and for strictures involving the bulbar urethra yields patency rates of 33.3-90% depending upon patient and stricture characteristics and patient satisfaction is high with all types of staged urethroplasty. | 3 |
| Lichen sclerosus is a relative contraindication for staged urethroplasty in the literature with lower long-term urethral patency rates of 52.2% compared to urethral patency rates of 64.3% in non-lichen sclerosis patients. | 3 |
| Up to 45.5% of men elect not to proceed to 2nd stage re-tubularisation after successful 1st stage. | 3 |
| Up to 19% of men required revision of their 1st stage urethroplasty. | 3 |
| Recommendations | Strength rating |
| Offer staged urethroplasty to men with complex anterior urethral stricture disease not suitable for single stage urethroplasty and who are fit for reconstruction. | Weak |
| Do not perform staged bulbar urethroplasty for lichen sclerosis if single stage urethroplasty is possible. | Weak |
| Consider staged procedure in patients unsure about perineal urethrostomy versus urethral reconstruction. | Weak |
| Warn men that staged urethroplasty may comprise more than two stages. | Weak |
6.3.2.3.3. Risk factors
Much effort has been made in attempting to identify risk factors affecting the success rate of urethroplasties. The evidence is not clear about age, obesity, aetiology or prior radiation or prior surgery. The only clear risk factors in multivariate analysis are the length and the site of the stricture, and success rates are better in shorter or bulbar strictures and worse in longer or penile strictures [317,344-346]. Patient should be informed about the higher risk in longer and penile strictures.
6.3.2.3.4. Risk factors for adverse outcomes
In four series specifically dedicated to risk factors for failure after urethroplasty using multivariate analysis, there is conflicting evidence about several factors (aetiology, comorbidity, stricture length, prior therapy) that might be predictive for failure after urethroplasty (Table 6.6). Advanced age does not appear to be a risk factor for urethroplasty failure in the majority of studies, with the exception of Viers et al., 2017 [347] retrospective case series which found that the risk for recurrence was significantly higher beyond the age of 60 (< 50 yrs 94%, > 70 yrs 74%) in 184 patients having a wide variety of urethroplasties. Previous radiation therapy was also found to be a risk factor for stricture recurrence in both Viers’ [347] retrospective case series and Ahyai’s 2015 series [348] – with only a 71% patency rate at a median follow-up of 29 months in those with previous radiotherapy. Based on these data, a clear and evidence-based recommendation cannot be formulated.
Table 6.6: Risk factors for failure after urethroplasty based on multivariable Cox regression analyses
| Study | N | Population | Comorbidity HR (95% CI) | Length HR (95% CI) | Aetiology HR (95% CI) | Prior stricture therapy HR (95% CI) |
Breyer et al. 2010 [349] | 443 | Mixed | NS | NS | NS | Prior DVIU: Prior urethroplasty: |
Kinnaird et al. 2014 [350] | 604 | Mixed | NS | > 5 cm: 2.3 (1.2-4.5) | Iatrogenic: LS: Infectious: | NS |
Chapman et al. 2017 [317] | 596 | Isolated bulbar strictures | Overall comorbidity: Obesity: | 1.2 (1.1-1.3) | Infectious: 3.7 (1.3-10.6) | NS |
Verla et al. 2020 [351] | 474 | Anterior strictures | NS | NS | NS | NS |
CI = confidence interval; HR = hazard ratio; LS = lichen sclerosus; N = number of patients; NR = not reported; NS = not significant.
6.3.2.4. Management of recurrence after bulbar urethroplasty
Kahokehr et al., [334] followed nearly 400 patients after urethroplasty and found a recurrence rate of 6% (n=25). Ninety-two percent of the failed cases were treated successfully with DVIU and only 8% needed another open reconstruction. However, they did not mention characteristics of the recurrent cases nor the duration of follow-up. Rosenbaum et al., [352] and Javali et al., [353] retrospectively analysed the outcomes of BMG FGU for ReDo urethroplasty in 51 and 21 patients, respectively, using the other cheek as donor side. Patency rates were 82-86%, which is in the range of primary cases.
Vetterlein et al., [354] compared primary (no previous open urethroplasty) vs. ReDo (previous open urethroplasty with BMG) vs. secondary (previous open urethroplasty without use of BMG) cases in a retrospective series of 534 patients with BMG FGU. The patency rates in primary and ReDo cases were comparable (87%) whilst the outcome in secondary cases was worse (71%).
A small series (n=37) reported on the use of EPA for revision surgery after failed urethroplasty in strictures of 2.1 (range 1-3.5) cm length on average. Patency rates using EPA after failed primary EPA (51%) and after any other technique of urethroplasty (49%) were 95 and 94% respectively with a mean follow-up of 30 months [321].
| Summary of evidence | LE |
| Buccal mucosa free graft urethroplasty after failed urethroplasty achieves the same patency rates as primary cases. | 3 |
| Recommendation | Strength rating |
| Use oral mucosa free graft urethroplasty for ReDo urethroplasty in case the of a long stricture. | Strong |
6.3.3. Urethroplasty for penobulbar or panurethral strictures
The possibilities for reconstruction are various and often include combinations of different techniques or grafts other than OMG. The patency rates are usually lower than in shorter reconstructions (Table 6.7). Hussein et al., [355] performed a RCT comparing skin grafts vs. skin flaps in strictures of mean length 15 cm and found no difference in patency rates (72% vs. 79%) or complications.
Warner et al., [305] performed a multi-institutional review in 2015 including 466 patients with stricture length > 8 cm and found an overall patency rate of 77.5%. As discussed previously, Kozinn et al., [257] reported on the outcome of staged urethroplasty in a cohort of which 54.9% had panurethral strictures (Table 6.7).
Kulkarni et al., [356] proposed a one-stage completely perineal approach with invagination of the penis and one-sided urethral dissection. After 59 months the overall patency rate was 83.7% in 117 men with a mean stricture length of 14 cm.
Another option in patients refusing or unfit for complex reconstructive surgery is PU (see section 6.3.4 Perineal urethrostomy).
Table 6.7: Study characteristics and patency rates of series on penobulbar strictures
| Author | Study | Length in cm (min, mean, range) | Technique | N | FU months (mean, range) | Patency |
| Hussein et al. 2011 [355] | RCT | NR, 15, 9-21 | Skin graft vs. flap | 37 | 36, 12-60 | 72 vs. 79% |
| Hussein et al. 2016 [357] | Prospective | NR, 8, NR | BM vs. skin dorsal onlay | 69 | 56, NR | 90 vs. 84% |
| Warner et al. 2015 [305] | Retrospective review | > 8, 12.5, 8-24 | BM/staged/skin | 466 | 20, 12-344 | 77.5% |
| El Dahshoury et al. 2009 [358] | Retrospective | NR, 18, 15-20 | Skin flap | 30 | 24, NR | 87% |
| Mathur et al. 2010 [359] | Retrospective | NR, 12, 8-16.5 | Tunica albuginea graft | 86 | 36, NR | 89% |
| Meeks et al. 2010 [360] | Retrospective | NR, 11, 4-24 | Abdominal skin graft | 21 | 28, 11-52 | 81% |
| Kulkarni et al. 2012 [356] | Retrospective | NR, 14 | BM dorsal onlay | 117 | 59, NR | 83.7% |
| Tabassi et al. 2014 [361] | Retrospective | NR, 14.4, NR | BM dorsal onlay | 117(37) | 19, NR | 84% |
| Xu et al. 2017 [301] | Retrospective | > 8, 12, 8-20 | BM/LM/combination | 81 | >12, 41, 15-86 | 83% |
| Alsagheer et al. 2018 [362] | Retrospective | > 8, 11.3 | BM onlay vs. skin flap | 50 | NR, 16, NR | 70 vs. 77% |
| Kozinn et al. 2013 [257] | Retrospective | NR, 9.6, 4-17 | Staged urethroplasty | 91 | 15, 12-69 | 90.1% |
BM = buccal mucosa; LM = lingual mucosa; FU = follow-up; N = number of patients; NR = not reported; RCT = randomised controlled trial.
| Summary of evidence | LE |
| Publications about panurethral urethroplasties generally come from high volume centres. | 4 |
| Different materials and techniques might be needed for reconstruction. | 3 |
| Recommendations | Strength rating |
| Offer panurethral urethroplasties in specialised centres because different techniques and materials might be needed. | Weak |
| Combine techniques to treat panurethral strictures if one technique is not able to treat the whole extent of the stricture. | Weak |
6.3.4. Perineal urethrostomy
6.3.4.1. Indications
Perineal urethrostomy offers a permanent or temporary solution for restoration of voiding in men with complex urethral stricture disease in whom:
- there are no further options to restore urethral patency either due to multiple previous failed urethroplasties [305,339] or multiple co-morbidities precluding a more expansive surgical undertaking after failed endoscopic management [363];
- there is a lack of certainty on behalf of the surgeon regarding the most appropriate form of urethroplasty for the patient;
- following urethrectomy and/or penectomy for cancer [364].
6.3.4.2. Types of perineal urethrostomy
Johanson described an inverted anterior scrotal funnel PU in 1953. This was later modified by Gil-Vernet and Blandy to utilise a posteriorly based scrotal flap. Both these techniques utilise an inverted U or lambda incision. The Gil-Vernet-Blandy PU has been further modified with the addition of dorsal and/or ventral free OMG augment to allow use of PU in men with strictures consequent to radiotherapy [365] or LS [259] and/or in men with PU stenosis or stricture extending into the proximal bulbar or membranous urethra (“augmented Blandy”) [363].
More recently, the ‘7 flap’ PU utilising a unilateral posteriorly based scrotal flap has been developed for use in the very obese, or in men of all BMI with stricture extension into the proximal bulbar or membranous urethra [366]. Initially this was performed with transection of the distal bulbar urethra but latterly the technique has been modified to a non-transection technique with loop mobilisation of the bulbar urethra (“loop PU”) [367]. The “7-flap” utilises a midline incision – which has been shown to have a significantly reduced side-effect profile in terms of superficial wound infection (1.9% c.f. 18.6%) and superficial wound dehiscence (11.9% c.f. 23.3%) than the inverted U or lambda incision [368,369] and may be associated with improved urethroplasty (and by inference PU) outcomes, at least in the short term (0% failure c.f. 6.2% failure at six months) [368]. Operative time is similar for all types of PU with mean operative time varying between 97.2 minutes to 112 minutes [364,370].
The utilisation of PU is increasing [371] – constituting 4.5% of 403 procedures for complex urethral stricture disease in a tertiary centre in 2008 and 38.7% in 2017 [372]. Perineal urethrostomy patients are generally older than those having urethroplasty with a median of 62.6 years of age for men having PU in Fuchs et al., 2018 series compared with a median of 53.2 years for men having anterior urethroplasty [372]. Between 18.7% and 73.4% of men having staged urethroplasty for complex anterior urethral stricture decline to proceed to 2nd stage re-tubularisation after a successful 1st stage and remain voiding from the PU of their 1st stage urethroplasty [257,339,342].
6.3.4.3. Outcomes
6.3.4.3.1. Patency rates
Patency rates of 70-95% at mean/median follow-up of 20–63 months have been described [305,339,347,363-366,370,372]. All reports are retrospective series – all of which are heterogenous in terms of indications and patients. There is consequently little data available to determine which is the best technique for PU.
McKibben et al., reported a patency rate of 92.9% in 42 patients for “7-flap” PU at median follow-up of 53.6 months, whilst they had a 100% patency rate with loop PU in twenty patients at a median follow-up of thirteen months [367].
Lumen et al., in 2015 reported a 74.3% patency rate for Johanson PU compared with an 87.5% patency rate for Gil-Vernet-Blandy PU (p=0.248), but with a significantly longer follow-up after Johanson PU (median 36 vs. nine months) [364]. Barbagli et al., published the largest series of PU patients to date – including 173 men (all of whom had been planned to have a staged urethroplasty for their complex anterior urethral stricture disease and 127 [73.4%] of whom declined to proceed with 2nd stage re-tubularisation). The median follow-up in this series was 62 months and the patency rate was 70% - confirming that patency rates for PU (and indeed for all urethroplasty [273,346]) reduce with time [339].
See supplementary Table S6.11 for further information.
6.3.4.3.2. Complications
Perineal urethrostomy complications occur in 2.5-11.4% of patients and include superficial wound dehiscence, scrotal abscess, UTI and urosepsis, bleeding, and transient scrotal pain and numbness [305,364,373]. The majority of complications are Clavien-Dindo grades 1 (2.9-18.8%) and 2 (0-2.9%). Grade 3 complications are rare and only occur in 5.7-6.2%. In the medium-term 22.2-30.8% of men with PU report post-micturition dribble [364].
6.3.4.3.3. Patient reported outcomes
Barbagli et al., reported that 168/173 (97.1%) of men were satisfied or very satisfied with the outcome of their Gil-Vernet-Blandy PU and would have the procedure again at median 62 months follow-up. Of these, 166/173 (95.9%) felt they had excellent or good results from their Gil-Vernet-Blandy PU, 145/173 (85%) felt it caused them no problems and 141/173 (82%) felt it caused their partner no problems [339]. The Trauma and Urologic Reconstructive Network of Surgeons (TURNS) collaborative group found no significant change in sexual function and a significant improvement in urinary symptoms following PU in a small group of patients [374], whilst Lumen et al., found satisfactory or acceptable International Prostate Symptom Score (IPSS) outcomes in 26/32 (81.25%) of men with Johanson or Gil-Vernet-Blandy PU at a median follow-up of 32 months and nine months, respectively.
McKibben et al., found a mean patient global impression of improvement (PGI-I) of 1.3 in nineteen patients with either loop PU or “7-flap” PU [367] at median 31 months follow-up.
6.3.4.3.4. Risk factors for patency failure of the perineal urethrostomy
Lichen sclerosus, trauma and infection urethral strictures have poorer outcomes from PU, with PU patency failure in 36.7-67% at a median 62 month follow-up [339,373]. Worse outcomes were also observed in patients with previous failed urethroplasty and multiple previous endoscopic and open treatments [339,364,365].
Barbagli et al., found that stricture length was inversely related to PU patency, as was patient age [339]. Conversely Viers et al., found outcomes worsened with age, reporting patency rates of 100% in men < 50 years old compared with 83% in men aged 60-69 years old [347]. Lopez et al., found increased risk of PU failure in men with ischaemic heart disease which would be a putative explanation for the age-related worsening of outcomes noted by Viers et al., [373].
Failure of PU is most commonly treated with surgical revision of PU using V-Y plasty, augmentation or complete ReDo but can also be managed with periodic dilatation or urinary diversion [339,363,364].
For further information see supplementary Table S6.11.
| Summary of evidence | LE |
| Perineal urethrostomy provides very good short- and long-term outcomes for men with complex urethral stricture disease. | 1a |
| Perineal urethrostomy (PU) provides very good short and long-term outcomes for men who are unable to have complex reconstruction due to co-morbidities. | 2b |
| All types of PU yield equivalent very good outcomes. | 4 |
| Augmented Gil-Vernet-Blandy or “7-flap” PU yield very good outcomes in men with extension of their urethral stricture disease into the proximal bulbar or membranous urethra. | 2 |
| “7-flap” PU yields very good results in obese men. | 3 |
| Recommendations | Strength rating |
| Offer perineal urethrostomy (PU) as a management option to men with complex anterior urethral stricture disease. | Strong |
| Offer PU for men with anterior urethral stricture disease who are not fit or not willing to undergo formal reconstruction. | Weak |
| Choose type of PU based on personal experience and patient characteristics. | Weak |
| Consider augmented Gil-Vernet-Blandy perineal urethrostomy or “7-flap” PU in men with proximal bulbar or membranous urethral stricture disease. | Weak |
| Consider “7-flap” urethroplasty in obese men. | Weak |
6.3.5. Posterior urethra
6.3.5.1. Non-traumatic posterior urethral stenosis
6.3.5.1.1. Treatment of non-traumatic posterior urethral stenosis
Several treatment modalities including conservative management (see section 6.1 Conservative options), endoluminal, open or minimally invasive surgical procedures are currently available, depending on patient’s goals and health status.
6.3.5.1.2. Endoluminal management of non-traumatic posterior urethral stenosis
6.3.5.1.2.1. Dilatation of non-traumatic posterior urethral stenosis
This can be done under loco-regional anaesthesia [375-379]. Dilatation is used for VUAS [375-380] or radiation induced BMS [381] and in the majority of reported cases, patients were not previously treated for their stricture (see supplementary Table S6.12). Patency rates vary widely between 0-89% [117,375-381]. The risk of de novo UI was low (0-11%) and no other complications were reported. It is of note that most series report on visually controlled dilatation [375-379] in VUAS without complete obliteration.
6.3.5.1.2.2. Endoscopic incision/resection of non-traumatic posterior urethral stenosis (Table 6.8)
Incisions can be performed at multiple locations according to surgeon’s preference [382]. However, aggressive incisions at the six and twelve o’clock positions should be avoided because of the risk of, respectively, rectal injury and urosymphyseal fistulation [189,383-385]. The risk of urosymphyseal fistulation is especially a concern after previous radiotherapy [386]. Result of bladder neck incision for VUAS are poorer after radiotherapy [387]. Direct vision internal urethrotomy is mainly performed in patients with primary or recalcitrant VUAS although one series performed it in a mix of patients with VUAS and BNS [388] and two series reported it for radiation-induced BMS [117,381]. Direct vision internal urethrotomy/dilatation for non-irradiated BMS are usually included in series reporting on anterior strictures (see section 6.2 Male endoluminal treatment of anterior urethral strictures). Patency after a 1st “cold/hot knife” DVIU ranges between 25-80% [375,376,380,382,388-393]. Laser incision yields a 69-100% patency rate [376,380,394,395]. In a retrospective and unbalanced series, La Bossiere et al., found better patency rates for laser incision as compared to dilatation, “cold knife” DVIU and transurethral resection (TUR) [376]. Redshaw et al., reported inferior patency rates for “cold knife” incision vs. “hot knife” incision followed by MMC for BNS (50 vs. 63%; p=0.03) [236] (see supplementary Table S6.13).
Urinary incontinence largely varies between 0 and 53% but some series have not assessed urinary continence before DVIU [389,391]. In series where pre- DVIU continence data were available, de novo urinary continence after DVIU ranges between 0% and 10% [375,380,390,392,394]. Noteworthy, of 21 patients that were incontinent pre-DVIU in the series of Giannarini et al., eleven (52%) patients became continent, and eight (38%) patients experienced improvement after DVIU [390]. As most recurrences will occur early [390,391], it is advised to wait for three to four months after DVIU [382,391,396] to proceed with incontinence surgery, if necessary, although others wait for twelve months [397]. The presence of recurrence must be ruled out by cystoscopy prior to incontinence surgery [382,391,396,397].
Another option is to resect the stenosis. Popken et al., reported a 47% patency rate with TUR for untreated VUAS and no patient suffered de novo SUI [392]. Kranz et al., compared the results of TUR in 87 and 60 patients with, respectively, VUAS after RP and BNS after TURP. After a median follow-up of 27 (range: 1-98) months, patency rate was 40.2% for VUAS and 58.3% for BNS (p=0.031). The rate of de novo incontinence was significantly higher in patients treated for VUAS compared to BNS (13.8 vs. 1.7%; p=0.011) [398]. There is conflicting evidence whether resection is associated with higher incontinence rate compared to incision. [379,399] Brodak et al., compared TUR by bipolar resection (n=22) with holmium laser incision and vaporisation (n=17). After a mean follow-up of 42 months, two (9.1%) and four (23.5%) patients suffered a recurrence with bipolar and laser resection respectively (p=0.37). After six months, patients treated with bipolar resection had a significant better Qmax compared to laser treatment (13 vs. 6.1 ml/s; p < 0.001) [395]. Bipolar plasma vaporisation produced an 82% patency rate at a mean 24-month follow-up in 28 patients with VUAS who previously failed endoscopic treatment [400].
Cut-to-the-light technique for a complete obliterative stricture is not advised because of the very-low likelihood of durable patency and for the risk of false passage towards the rectum [396,401,402].
Repeat DVIU was often able to stabilise the stricture [117,375,376,381,388-390,398], but ultimately 6-10% required urinary diversion [391] or chronic suprapubic cystostomy [381,388].
Transurethral resection can be performed for prostatic obstruction due to sloughing after high-energy treatments (HIFU, cryoablation) [100]. Transurethral resection for obstructive necrotic debris after radiotherapy is possible but is of limited role. Risk of recurrence is 50% and risk of de novo UI is 15-25% [100].
Table 6.8: Results of endoluminal incision/resection for posterior non-traumatic stenosis
| Study | Modality | Type | N | Previous treatment (%) | FU (months) | Patency° (%) | Urinary incontinence (%) | Complications (%) |
| Merrick et al. [381] | Dilatation / “Cold knife” DVIU | Radiation-induced BMS | 29 | 0 | NR | 69 | NR | NR |
| Sullivan et al. [117] | Dilatation (n=15) / “Cold knife” DVIU (n=20) | Radiation-induced BMS | 39 | 0 | 16 (2-48) | 51 | 11 | NR |
| Brede et al. [391] | “Cold knife” DVIU | VUAS | 63 | Dilation: 33 Incision: 38 Both: 29 | 11 (1-144) | 73 | 52* | NR |
| Yurkanin et al. [389] | “Cold knife” DVIU | VUAS | 61 | Dilatation: 100 | 31 (1-77) | 87 | 12** | NR |
| Giannarini et al. [390] | “Cold knife” DVIU | VUAS | 43 | 0 | 48 (23-80) | 74 | 0 | NR |
| Ramchandani et al. [375] | “Cold knife” DVIU | VUAS | 10 | 0 | NR | 80 | 10 | 0 |
| Hayashi et al. [380] | “Cold knife” DVIU | VUAS | 6 | Dilatation: 100 | NR | 50 | NR | NR |
| Holmium laser DVIU | VUAS | 3 | Dilatation + DVIU: 100 | 11-37 | 100 | 0 | NR | |
| Lagerveld et al. [394] | Holmium laser DVIU | VUAS | 10 | None: 40 Endoscopic (dilatation +/- DVIU +/- ISD): 60 | 18 (3-29) | 100 | 0 | 0 |
| Ramirez et al. [388] | “Hot knife” DVIU | VUAS: 74% BNS: 26% | 50 | None: 22 | 16 | 72 | 9 | NR |
| Gousse et al. [393] | “Hot knife” DVIU | VUAS | 15 | None | 15 (6-26) | 80 | 100*** | NR |
| Bang et al. [382] | “Hot knife” DVIU | VUAS | 37 | NR | 13 (2-33) | 65 | 100*** | NR |
| Popken et al. [392] | “Cold knife” DVIU | VUAS | 6 | None | 12-72 | 50 | 0 | NR |
| TUR | VUAS | 15 | None | 47 | 0 | NR | ||
| Kranz et al. [398] | TUR | VUAS | 87 | NR | 27 (1-98) | 40.2 | 13.8 | NR |
| TUR | VUAS | 60 | NR | 58.3 | 1.7 | NR | ||
| Brodak et al. [395] | TUR (bipolar) | BNS | 22 | DVIU: 45 | 42 (14-72) | 91 | NR | NR |
| Holmium laser DVIU | VUAS | 17 | DVIU: 12 | 76 | NR | NR | ||
| Ozturk et al. [400] | TUR (bipolar) | VUAS | 28 | Dilatation: 75 DVIU: 25 | 24 (6-66) | 82 | 0 | 0 |
| LaBossiere et al. [376] | Holmium laser DVIU | VUAS | 70 | NR | 10 | 69 | NR | NR |
| “Cold knife” DVIU | VUAS | 8 | NR | 25 | NR | NR | ||
| TUR | VUAS | 36 | NR | 39 | NR | NR |
BNS = bladder neck stenosis; DVIU = direct vision internal urethrotomy; FU = follow-up; ISD = intermittent self-dilatation; NR = not reported; TUR = transurethral resection; VUAS = vesico-urethral anastomosis stricture.°patency rate after 1st endoluminal treatment evaluated in the study.
* requiring incontinence surgery (artificial urinary sphincter or male sling).
** slightly problematic urinary incontinence by questionnaire post DVIU (no data on pre DVIU continence).
***all incontinent pre-operatively.
6.3.5.1.2.3. Post-dilatation/direct vision internal urethrotomy strategies for non-traumatic posterior urethral Stenosis
6.3.5.1.2.3.1.Intermittent self-dilatation for non-traumatic posterior urethral stenosis
As for anterior strictures, ISD can be offered to patients for recurrent posterior stenosis after dilation/DVIU to stabilise the stenosis. This is especially relevant for patients unfit/unwilling to undergo surgery or in patients with radiation-induced BMS [117,376,381,403]. Although ISD may be acceptable to many urologists and patients, it usually is associated with a reduced QoL and poor patient compliance [33].
6.3.5.1.2.3.2.Intralesional injections for non-traumatic posterior urethral stenosis
In order to stabilise the luminal fibrosis and consequently to reduce the risk of recurrence, injection of antifibrotic agents at the time of endoluminal treatment has been proposed. The majority of patients in these studies were patients with recalcitrant/recurrent non-obliterative VUAS/BNS. Two series used corticosteroids [379,396], whilst the others used MMC [397,401-405]. Patency rates with corticosteroid injections range between 50-100% [379,396]. Patency rates with MMC vary between 50-94% [397,404-406]. No trials comparing endoluminal treatment with or without adjuvant intralesional injections were identified.
See supplementary Table S6.13 for further information.
Complications are low across most studies, but all studies were retrospective in nature. Redshaw et al., also reported grade 3 complications in four out of 55 (7%) patients, including osteitis pubis (n=2), bladder neck necrosis (n=1) and rectourethral fistula (n=1) in one multi-institutional study [404]. Three of these patients ultimately required urinary diversion, with additional faecal diversion in one patient [404]. Given the severity of these complications, although rare, MMC should not be used outside the framework of a clinical trial [407].
6.3.5.1.2.3.3.Urethral stent for non-traumatic posterior urethral stenosis
Stents have been used anecdotally in the posterior urethra [247,248,376]. Patency rates are relatively low (47-60%) [247,248,376] at the cost of a high-risk for UI (19-82%) [247,248].
| Summary of evidence | LE |
| For non-obliterative VUAS and radiation-induced BMS, visually controlled dilatation and DVIU yield a patency rate of respectively 0-89% and 25-100% with a low complication rate. It can be performed under loco-regional anaesthesia. | 3 |
| During DVIU, deep incision might provoke injury to the rectum at the six o’ clock position and might provoke uro-symphyseal fistulation at the twelve o’clock position. | 3 |
| For BNS, TUR and “hot-knife” incision yield a patency rate of respectively 58.3 and 72% with a low complication rate. | 3 |
| Repeat endoluminal treatments in non-obliterative VUAS, radiation-induced BMS or BNS can stabilise the posterior stenosis and are easy to perform compared to reconstructive surgery. | 3 |
| Any form of endoluminal treatment might be associated with de novo UI (up to 25%) or worsening of existing UI (up to 15%). | 3 |
| Vesico-urethral anastomosis stricture, BMS and BNS with complete obliteration are not included in present series and endoluminal treatment is unlikely to be successful. | 3 |
| Urethral stents at the posterior urethra have a rather low patency rate (47-60%) and incontinence rate (19-82%). | 3 |
| Recommendations | Strength rating |
| Perform visually controlled dilatation or direct vision internal urethrotomy (DVIU) as 1st line-treatment for a non-obliterative vesico-urethral anastomosis stricture (VUAS) or radiation-induced bulbomembranous strictures (BMS). | Weak |
| Do not perform deep incisions at the six and twelve o’ clock position during DVIU for VUAS or radiation-induced BMS. | Strong |
| Perform transurethral resection (TUR) or “hot-knife” DVIU as 1st line-treatment for patients with non-obliterative bladder neck stenosis (BNS) after surgery for benign prostatic obstruction. | Strong |
| Perform repeat endoluminal treatments in non-obliterative VUAS or BNS in an attempt to stabilise the stricture. | Weak |
| Warn patients about the risk of de novo urinary incontinence (UI) or exacerbation of existing UI after endoluminal treatment. | Weak |
| Do not perform endoluminal treatment in case of VUAS, BMS and BNS with complete obliteration. | Strong |
| Do not use stents for strictures at the posterior urethra. | Weak |
6.3.5.1.3. Lower urinary tract reconstruction for non-traumatic posterior urethral stenosis
If endoluminal treatment (repeatedly) fails or in case of a completely obliterated posterior stenosis [401,402,408,409], lower urinary tract (LUT) reconstruction may be considered in fit patients motivated to undergo surgery (Figure 6.1). The choice of LUT reconstruction will depend upon the length, location, calibre and aetiology of the stenosis, continence status, bladder function, previous radiotherapy, patient’s preference, and surgeon’s expertise.
Figure 6.1: Options for lower urinary tract reconstruction of non-traumatic posterior urethral obstruction (stenosis/stricture) 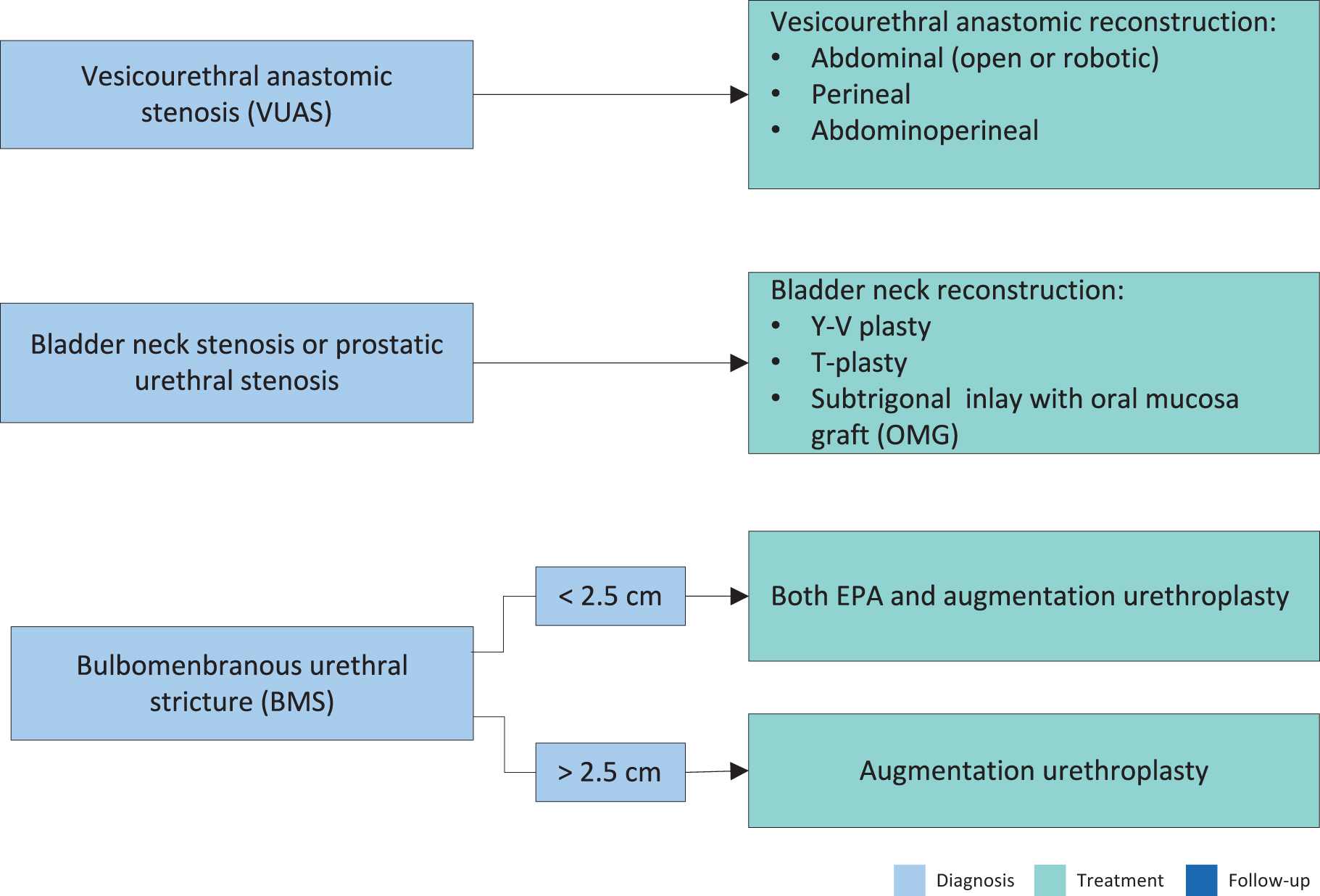
6.3.5.1.3.1. Redo vesico-urethral anastomosis for vesico-urethral anastomotic stenosis after radical prostatectomy
After excision of the stenosis, ReDo vesico-urethral anastomosis (ReDo VUA) can be performed. This may be performed via a retropubic, perineal, combined abdominoperineal or robot-assisted approach. Nikolavsky et al., proposes a retropubic approach for VUAS involving the bladder neck, a perineal approach for short VUAS with intact bladder neck and an abdominoperineal approach for long segment (> 3 cm) VUAS with bladder neck involvement [408]. The ReDo VUA must be performed in a tension-free fashion which can be achieved either by mobilisation of the bladder (retropubic approach), mobilisation of the bulbar urethra with corporal splitting and inferior pubectomy if necessary (perineal approach) or both (abdominoperineal approach) [408,410]. Dinerman et al., reported a robot-assisted abdominoperineal approach in a case with 4.5 cm long complete obliteration [411]. Kirshenbaum et al., reported a pure robot-assisted abdominal approach. Regardless of the approach, the procedure is technically demanding due to the location deep under the pubic symphysis, and the proximity of the external sphincter [410]. As a consequence, surgical morbidity must be considered. As most patients with VUAS were healthy enough to undergo RP, most patients will likewise remain fit and eligible for VUAS surgical reconstruction [408,410].
Table 6.9: Outcomes of redo vesico-urethral anastomosis
| Study | N | Approach (%) | Previous RT (%) | FU (months) | Length (cm) | Patency (%) | Incontinence (%) | Complications (%) |
| Nikolavsky et al. [408] | 12 | Perineal: 25 Abdominal: 67 Abdominoperineal: 17 | 25 | 76 (14-120) | 2.5 (1-5) | 67 | 58 | Persistent extravasation due to anastomotic dehiscence grade 3b: 8.3 (prior RT) |
Mundy et al. [410] | 17 | Transperineal | 0 | NR | NR | 88 | 100 | NR |
| 6 | 100 | NR | NR | 67 | 100 | NR | ||
Schuettfort et al. [412] | 22 | Transperineal | 0 | 45 (4-77) | NR | 91 | 100* | Rectal injury: 4 Lower leg paresthesia: 4 |
| 1 | 100 | NR | 0 | 100* | ||||
Pfalzgraf et al. [413] | 20 | Retropubic | NR | 63 (15-109) | NR | 60 | 65** | UTI: 5 Fever: 5 Renal failure: 5 (all grade 2) |
Giudice et al. [414] | 10 | Perineal: 5 Abdominal: 4 Combined: 1 | NR | 30 (4-106) | NR | 80 | 70 | NR |
Dinerman et al. [411] | 1 | Robot-assisted abdominoperineal | 0 | 12 | 4.5 | 100 | 0*** | 0 |
| Kirshenbaum et al. [409] | 5 | Robot-assisted abdominal (±VY-plasty) | 0 | 14 (5-30-) | NR | 60 | 0 | Pubovesical fistula: 20 grade 3b |
FU = follow-up; NR = not reported; RT = radiotherapy; UTI = Urinary tract infection.
* incontinent before ReDo VUA.
** de novo incontinence in four out of eleven patients.
***social continent (1 pad/day).
ReDo VUA in non-irradiated patients yields patency rates of 60-91% (Table 6.9) [408-410,412-414]. Prior radiotherapy is a risk factor for failure [410,412]. In addition, radiation-induced bladder toxicity might provoke reduced bladder capacity, low bladder compliance, bladder spasms and pain, and urethral necrosis making reconstruction futile (see below) [386,410,415]. ReDo VUA should only be done in patients with adequate bladder function and in the absence of (peri)-urethral pathology (urethral necrosis, calcification, fistulation). Flaps (gracilis flap, peritoneal flap) to support and protect the anastomosis may be beneficial in irradiated patients [408].
With the transperineal approach, UI is inevitable, as this approach disrupts the external sphincter [409,410,412,414]. With the retropubic approach, Pfalzgraf et al., reported de novo incontinence in only four out of eleven (36%) patients [413]. In the series of Nikolavsky et al., where a retropubic approach was predominantly used, incontinence rate was 58% [408]. Kirshenbaum et al., reported no incontinence in five patients treated by robot-assisted retropubic approach [409]. Giudice et al., reported incontinence in one out of four patients treated with the retropubic approach [414]. Therefore, some authors [100,408,409] have proposed a preference for the retropubic approach in patients with good pre-operative urinary continence, although both approaches have never been directly compared for UI. In addition, the lack of perineal dissection by a retropubic approach will preserve the perineal anatomy and vascularisation which makes subsequent artificial urinary sphincter (AUS) less demanding [409]. Artificial urinary sphincter implantation should be deferred because of the risk of VUAS recurrence and difficulty of treating any recurrent VUAS with the cuff of the AUS in place [391,410]. The exact timing of AUS placement is not consensual in the literature but most advise waiting at least three to six months to ensure stability of the VUA patency [386,407,410,412].
Due to the complexity of this pathology the EAU Urethral Strictures Panel advises that VUAS reconstruction should be performed only in experienced high-volume centres, particularly after prior radiotherapy or other energy ablative treatments.
| Summary of evidence | LE |
| ReDo VUA has patency rates of 60-91% in non-irradiated patients and 67% in irradiated patients with obliterative VUAS or VUAS refractory to endoluminal treatment. | 3 |
| Urinary incontinence is inevitable after transperineal ReDo VUA. Artificial urinary sphincter placement can be offered after three to six months if patency of ReDo VUA is ensured. | 3 |
| De novo incontinence with retropubic ReDo VUA is 0-58%. | 3 |
| Recommendations | Strength rating |
| Perform ReDo vesico-urethral anastomosis (VUA) in non-irradiated patients and irradiated patients with adequate bladder function with obliterative vesico-urethral anastomosis stricture or vesico-urethral anastomosis stricture refractory to endoluminal treatment. | Weak |
| Warn patient that urinary incontinence (UI) is inevitable after transperineal ReDo VUA and that subsequent anti-UI surgery might be needed in a next stage, after at least three to six months. | Strong |
| Offer ReDo VUA by retropubic approach if the patient is pre-operatively continent. | Weak |
6.3.5.1.3.2. Posterior stenosis after surgery for benign prostatic obstruction
6.3.5.1.3.2.1.Bladder neck reconstruction for bladder neck stenosis after surgery for benign prostatic obstruction
The bladder neck is augmented by advancement of local bladder flaps (Y-V or T-plasty) with or without resection of scar tissue. They are used for BNS refractory to endoscopic treatments [409,416-418]. Patency rates vary between 83-100% with fourteen to 45 months follow-up [409,416-418]. There is a trend to perform bladder neck reconstruction by minimally invasive approach (laparoscopic, robot-assisted) [409,417,418]. De novo incontinence rate ranges from 0-14% [409,416-418]. Satisfaction among patient is high with 88.5% of patients stating that they are pleased with the surgery, with an improvement of QoL in 75% of patients [416,418]. Recently, a robot-assisted augmentation technique with subtrigonal buccal mucosa inlay has been successfully reported in a case report, but this technique requires further investigation [419].
See supplementary Table S6.14 for further information.
6.3.5.1.3.2.2.Bulbomembranous strictures after surgery for benign prostatic obstruction
Bulbomembranous urethral strictures (BMS) after TURP or simple prostatectomy are managed as bulbar strictures and can be treated by EPA or augmentation urethroplasty with a graft, taking into account the length and tightness of the stricture [82]. Kulkarni et al. reported a similar patency rate for dorsal and ventral onlay urethroplasty (resp. 81.8% versus 84.6% after mean follow-up of 14 months) [420]. As reconstruction is in the proximity of the external sphincter and the bladder neck was already damaged during BPO surgery, the risk of incontinence (up to 25%) is present [82].
| Summary of evidence | LE |
| Bladder neck reconstruction with Y-V or T-plasty for treatment refractory BNS has patency rates of 83-100%. | 3 |
| Incontinence occurs in up to 14% with bladder neck reconstruction and up to 25% after reconstruction of BMS after previous surgery for BPO. | 3 |
| Recommendations | Strength rating |
| Perform bladder neck reconstruction with Y-V or T-plasty for treatment refractory bladder neck stenosis (BNS). | Weak |
| Warn patients about de novo urinary incontinence after reconstruction for BNS or bulbomembranous urethral strictures with previous benign prostatic obstruction surgery as aetiology. | Strong |
6.3.5.1.3.3. Radiation/high-energy induced posterior strictures
6.3.5.1.3.3.1.Bulbomembranous strictures secondary to radiation/high energy sources
The major challenge in treating radiation-induced strictures is the consequent tissue damage with impaired healing capacity, involving not only the stricture itself but also the adjacent proximal and distal areas of the scar [421]. Additionally, proximity of the stricture to the external sphincter can further complicate surgery [421]. Due to these challenges, patients with radiation-induced BMS have long been considered poor candidates for urethral reconstruction and have been treated with urinary diversion if endoscopic treatments failed or were not possible [421].
Most radiation-induced BMS are short and in these cases, EPA is possible avoiding the use of a graft or a local flap in an area of poor vascular health. However, EPA will not be possible for BMS with a long bulbar segment and in these cases, augmentation urethroplasty will be necessary despite the aforementioned concerns. A systematic review reported a pooled patency rate of 80% with no significant differences between type of urethroplasty (EPA versus augmentation urethroplasty). Stress UI was reported in 19% of cases [421]. Rourke et al., reported no significant differences between EPA and augmentation urethroplasty regarding de novo UI (26 vs. 25%; p=1), new onset ED (35 vs. 0%; p=0.06) or other adverse events (30% vs. 33%; p=1) [422].
6.3.5.1.3.3.2.Prostatic strictures secondary to radiation/high energy sources
Radiotherapy and high-energy modalities (cryoablation, HIFU) might provoke prostatic necrosis, sloughing and obstruction [100]. Cases refractory to TUR and with good bladder capacity might be salvaged by prostatectomy taking into account the morbidity associated with salvage RP (rectal injury, VUAS, incontinence) [100,423]. Mundy et al., treated nine patients with patency in six, (67%) and one (11%) needing an AUS for severe incontinence [410].
Cases with impaired bladder function, urethral necrosis and/or peri-urethral pathology should be considered for supravesical diversion, especially if a suprapubic catheter is not tolerated due to bladder pain or spasms
[386,407,410,415].
Recently, a “pull-through” procedure has been reported as an alternative to cutaneous diversion for reconstruction of the devastated posterior urethra associated with a defunctionalised bladder after radiation where tissue vascularity and quality is poor [424]. This novel technique of total LUT reconstruction combines salvage cystectomy, ileal neobladder formation and urethral pull-through. An AUS was implanted in a second stage. All eight patients maintained a patent posterior urethra after a median follow-up of 58 (range 16-84) months. Five patients experienced low-grade complications after the first stage, but no high-grade complications were reported. Four out of eight (50%) patients experienced cuff erosion with need for removal and subsequent reimplantation. After a median of two revision surgeries (range 0 to 4), all patients achieved social continence enhancing QoL [424]. This technique requires further validation before its use can be recommended.
| Summary of evidence | LE |
| Patency rates of urethroplasty for radiation-induced BMS is 80% with no significant differences between EPA and augmentation urethroplasty. | 3 |
| Radiation-induced BMS longer than 2-2.5 cm are rarely amenable for EPA. | 3 |
| De novo incontinence and new onset ED after urethral surgery for radiation-induced BMS are reported in respectively 19-26% and 0-35% of cases. | 3 |
| Salvage prostatectomy can achieve patency in 67% of patients for prostatic strictures after irradiation or high-energy treatments but morbidity is substantial. | 4 |
| Recommendations | Strength rating |
| Use either excision and primary anastomosis (EPA) or augmentation urethroplasty for short (< 2.5 cm) radiation-induced bulbomembranous strictures (BMS) refractory to endoscopic treatment depending on surgeon’s experience. | Weak |
| Perform augmentation urethroplasty for long (> 2.5 cm) radiation-induced BMS. | Weak |
| Warn patients about the risk of de novo incontinence and new onset erectile dysfunction after urethroplasty for radiation-induced BMS. | Strong |
| Offer salvage prostatectomy in motivated and fit patients with adequate bladder function in case of a prostatic stricture due to irradiation or high-energy treatment. | Weak |
6.3.5.1.4. Extirpative surgery and urinary diversion for non-traumatic posterior urethral stenosis
In complex and/or recurrent cases [408], LUT reconstruction is not possible or not indicated due to severe necrosis, calcification and significant morbidity, especially severe pain [407]. Intractable haematuria or fistulation might be other reasons to abandon the urethral outlet. Typically, the patient has a history of pelvic irradiation or high energy prostate cancer treatment and several previous attempts to achieve cure. Moreover, and equally important, any of the options used to deal with a devastated posterior urethra are dependent upon good bladder capacity, compliance and function allowing for bladder preservation as well as healthy distal ureters [386,407]. The last resort therapeutic option is urinary diversion (continent or incontinent) with or without cystectomy [410,415]. Different techniques have been described and the choice between them largely depends on the bladder capacity, presence of local symptoms, performance status and expectations of the patient. Cystectomy during urinary diversion is able to palliate symptoms of intractable bladder pain, spasms and haematuria which are especially prevalent after pelvic radiotherapy [425-428]. The satisfaction rate was reported to be 100% and the overwhelming majority of patients would have undergone this extirpative surgery an average of thirteen months sooner in a study of fifteen patients by Sack et al. [429]. In a report by Faris et al., 27% of the patients also required bowel diversion due to intractable gastrointestinal morbidity, highlighting the complexity of this pathology [415].
| Summary of evidence | LE |
| Urinary diversion can improve QoL in patients with a devastated lower urinary tract with a high satisfaction rate. | 3 |
| Cystectomy is able to palliate symptoms of intractable bladder pain, spasms, and haematuria. | 3 |
| Recommendations | Strength rating |
| Perform urinary diversion in recurrent or complex cases with loss of bladder capacity and/or incapacitating local symptoms. | Weak |
| Perform cystectomy during urinary diversion in case of intractable bladder pain, spasms and/or haematuria. | Weak |
6.3.5.2. Post-traumatic posterior stenosis
The acute and early management of PFUIs is discussed in the EAU Guidelines on Urological Trauma. A non-obliterative stenosis is the result of a partial injury at the membranous urethra or occurs after unsuccessful early realignment of a partial or complete injury. An obliterative stenosis is the consequence of a complete injury with a distraction defect between the ruptured urethral ends. The gap between these ends fills up with dense fibrotic tissue [11].
The deferred management of PFUI is at earliest three months after the trauma. After that period, the pelvic haematoma has nearly always resolved, the prostate has descended into a more normal position, the scar tissue has stabilised [430] and the patient is clinically stable and able to lie down in the lithotomy position [430].
6.3.5.2.1. Endoluminal treatment for post-traumatic posterior stenosis
6.3.5.2.1.1. Endoluminal treatment as primary treatment for post-traumatic posterior stenosis
Endoluminal treatment (dilation, DVIU) of an obliterative stenosis using the cut-to-the light principle will not be successful [46] and has a risk of creating a false passage towards the bladder base or rectum [431]. For a non-obliterative, short (< 1.5 cm) stenosis, one attempt of endoluminal treatment (endoscopic incision or dilation) can be performed. Kulkarni et al., reported a 92.3% and 96.5% stricture-free rate with “cold knife” and holmium laser urethrotomy, respectively (median follow-up respectively 61 and 57 months) [432]. These results are challenged by Barbagli et al., who reported a 51% stricture-free rate with holmium laser urethrotomy but with no data on length of follow-up available [433]. Cai et al., compared patient outcomes between bipolar plasma vaporisation and “cold knife” DVIU in 53 patients with posterior traumatic (80%) and iatrogenic (20%) urethral strictures with significantly different stricture-free rates of 81.5% vs. 53.8% at a mean follow-up of 13.9 months, respectively [434]. No severe complications were reported in either group. A statistically significant shorter operative time was found in the bipolar group [434]. Barratt et al,. calculated a composite stricture-free rate of 20% after all types of endoscopic treatments (but with a mix of obliterative and non-obliterative stenoses) [46]. De novo UI was reported in 4% of cases [46]. Repetitive endoluminal treatments are unlikely to be curative and must be discouraged as this delays the time to definitive cure and can lead to more complications [435,436].
6.3.5.2.1.2. Endoluminal treatment after failed urethroplasty for post-traumatic posterior stenosis
In case of a non-obliterative and short (< 1 cm) recurrence after failed urethroplasty, endoluminal treatment can be performed [437]. Although a 1st and 2nd DVIU can be successful with a stricture-free rate of 22.9-77.3% and 0-60% respectively, three or more incisions are never successful (see supplementary Table S6.16) [437-440]. Therefore, repetitive endoluminal treatments (dilations and/or endoscopic incisions) can only be considered as a palliative option [441].
| Summary of evidence | LE |
| Endoluminal treatment of obliterative stenoses is not successful and may create false passages towards bladder or rectum. | 3 |
| A 1st DVIU has stricture-free rates of 22.9-77.3% for a short and non-obliterative recurrence after excision and primary anastomosis. | 3 |
| Three or more endoscopic incisions are never successful for recurrence after excision and primary anastomosis. | 3 |
| Recommendations | Strength rating |
| Do not perform endoscopic treatment for an obliterative stenosis. | Strong |
| Perform one attempt at endoluminal treatment for a short, non-obliterative stenosis. | Weak |
| Do not perform more than two direct vision internal urethrotomies and/or dilatations for a short and non-obliterative recurrence after excision and primary anastomosis for a traumatic posterior stenosis if long-term urethral patency is the desired intent. | Weak |
6.3.5.2.2. Urethroplasty for post-traumatic posterior stenosis
In view of the complexity and difficulty of urethroplasty and the fact that the best results are obtained with its first attempt, this surgery must be performed in high-volume centres [442]. It has been calculated that to achieve and maintain sufficient experience in the reconstruction of PFUI, one centre per twelve million inhabitants is sufficient (for well-resourced countries) [443].
6.3.5.2.2.1. First urethroplasty for post-traumatic posterior stenosis
6.3.5.2.2.1.1.Indication and technique of urethroplasty for post-traumatic posterior stenosis
Progressive perineal EPA is the standard treatment for an obliterative stenosis and for a non-obliterative stenosis as first attempt, or after failure of primary endoluminal treatment [46,444].
Although both a midline and inverted U-incision are possible to gain access to the posterior urethra, a midline incision is associated with a significant reduction in trauma to the superficial perineal and posterior scrotal nerves and vessels, in the rate of surgical site infections (3.1% vs. 16.4%) and reduced length of hospitalisation [369].
A combined transpubic abdomino-perineal approach is only necessary in complicated cases such as those with associated para-urethral bladder base fistula, trauma-related recto-urethral fistula, and bladder neck injury [431]. Total pubectomy during transpubic abdomino-perineal reconstruction has a higher complication rate (bleeding, pelvic instability, dead space) compared to partial (superior or inferior) pubectomy with no gain in surgical exposure [445]. Although also considered complex situations, iatrogenic recto-urethral fistula (after misdirected endoscopic treatment), traumatic recto-urethral fistula < 5 cm from the anus, UCF and urinoma cavity can usually be corrected by a progressive perineal approach only [431,446].
6.3.5.2.2.1.2.Patency rate after urethroplasty for post-traumatic posterior stenosis
The overall patency rate after deferred EPA is 85.7% [46]. Complete excision of scar tissue is a strong predictor for freedom of stricture whereas number (3-5 vs. 6-7) and size (3.0 vs. 4.0) of sutures are not [447]. A retrospective study showed an improved patency rate after eversion of the urethral mucosa of both urethral ends before anastomosis (“valgus urethral mucosa anastomosis”) [448], but this finding has yet to be confirmed in a prospective fashion.
To preserve the antegrade arterial inflow of the bulbar urethra and reduce the surgical trauma of “classic” deferred EPA, bulbar artery sparing EPA has been described [449]. Initial patency rates vary between 88.5-100% with 20-45 months of follow-up (see supplementary Table S6.17) [449-451]. Xie et al., only used this technique for distraction defects less than 2.5 cm [451]. No evidence exists to date whether bulbar artery sparing EPA is superior to the “classic” EPA in terms of patency rate and potency and continence rates.
In case of a very deep location of the proximal urethral end that makes anastomotic suturing impossible, Badenoch described a pull-through technique which has a 33.3-96.5% patency rate after 43-126 months of follow-up (see supplementary Table S6.18 for further information) [432,452,453]. With the aim to reduce stricture recurrence, Wong et al., advise a 1.5 cm segment overlap of the bulbar stump within the prostatic urethra during the pull-through technique [452]. To facilitate the suturing at the proximal part of the urethra located deep under the pubic bone, the robotic approach is under exploration but there is no evidence so far of improved outcome with this approach [454].
6.3.5.2.2.1.3.Sexual function, urinary continence, and rectal injury after urethroplasty for post-traumatic posterior stenosis
Regarding erectile function, a prospective study by Hosseini et al., found no significant difference before, and three or six months after EPA for posterior traumatic stenosis [455]. Another prospective study by Tang et al., also demonstrated no significant overall change in ED after urethroplasty. However, in the subgroup of patients with pre-operative non-vascular ED, a significant post-operative increase in ED was observed [456]. A meta-analysis of retrospective studies showed a significant decline of the rate of ED from 43.27% before to 24.01% after posterior urethroplasty (p < 0.001) [457]. Assessment of erectile function and its definitive treatment (e.g., penile prosthesis) should be performed two years after the trauma because of the potential return of normal erectile function within that time [458,459].
After deferred EPA, antegrade ejaculation is present in 98.3-100% of cases [460,461]. Decreased ejaculatory volume and/or diminished ejaculatory force were reported in 17.2-18.7% of cases but it cannot be assessed whether this is due to the trauma or due to the surgery [460,461].
Continence after PFUI and urethroplasty is generally attributed to a competent bladder neck [46]. On the other hand, as most ruptures occur at the bulbomembranous junction just below the external sphincteric mechanism, at least a part of the external sphincter mechanism can be spared during urethroplasty [462]. Therefore, incontinence is rare with deferred EPA (6.8-8.5%) and is usually due to incompetence of the bladder neck although an incompetent bladder neck will not necessarily result in incontinence after urethroplasty [46,462,463].
Rectal injury is a relatively rare (0-10.2%) but severe complication after deferred EPA (see supplementary Table S6.19) [430,438,445,463-467]. The risk of rectal injury tends to be higher in complicated cases or cases with previous urethral manipulations [430,468,469].
6.3.5.2.2.2. ReDo-urethroplasty for post-traumatic posterior stenosis
In case of a recurrent stenosis, a repeat (“ReDo”) urethroplasty is possible. In the majority of cases, especially if not all consecutive length-gaining manoeuvres have been used during the 1st EPA, another EPA can be performed [463,467,468,470-472]. The Badenoch pull-through technique is again an option if no adequate mucosa-to-mucosa suturing is possible (See supplementary Table S6.18) [452,453]. In case of excessive dead space after resection of the fibrosis, gracilis muscle [469] or omental flaps (laparoscopically harvested if urethroplasty was performed using perineal approach only) [431,465] have been advised to fill up this space and support the anastomosis. These flaps, or alternatively bulbospongious muscle or local subcutaneous dartos flaps, are also useful to separate the suture lines in case of a concomitant recto-urethral fistula [431,442,446,469]. If the urethra cannot be anastomosed in a tension-free fashion, despite the aforementioned manoeuvres, or in cases of ischemic narrowing/necrosis of the bulbar urethra, options are a tubed preputial island flap, staged BMG urethroplasty with flap, staged buccal mucosa dartos flap, radial forearm free flap urethroplasty or entero-urethroplasty [442,467,471,473]. In case of entero-urethroplasty, the sigmoid colon is preferred above ileum (which is in turn better than stomach) because of the proximity of the vascular pedicle to the perineum. Entero-urethroplasty should only be done in the presence of a competent bladder neck because subsequent implantation of an AUS is nearly impossible [473].
Patency rate of different types of ReDo-urethroplasty varies between 50-100% (Table 6.11) [442,463,467,468,471,473]. An alternative is to abandon the normal urinary outlet and opt for Mitrofanoff-vesicostomy, PU (if local perineoscrotal skin is suitable) or permanent suprapubic diversion [467,473].
Table 6.10: Outcome of different types of ReDo-urethroplasty
| Study | Type | N | Follow-up (months) | Patency rate |
| Bhagat et al. [471] | Progressive perineal EPA | 28 | 29 (12-108) | 36 (83.72%) |
| Transpubic EPA | 12 | |||
| Tubed preputial flap | 1 | |||
| Staged BMG + local flap | 2 | |||
| Fu et al. [468] | Progressive perineal EPA | 55 | 36 (18-47) | 33 (60%) |
| Garg et al. [467] | Progressive perineal EPA | 40 | 31 ± 11 | 30 (75%) |
| Transpubic EPA | 2 | 25 | 2 (100%) | |
| Tubed preputial flap | 1 | 25 | 1 (100%) | |
| Staged BMG + local flap | 2 | 17 | 1 (50%) | |
| Radial forearm free flap | 1 | 15 | 1 (100%) | |
| Sa et al. [463] | Progressive perineal EPA | 102 | 35 (6-63) | 93 (91.2%) |
| Kulkarni et al. [442] | Progressive perineal EPA | 541 | 68 (12-240) | 412 (79.1%) |
| Tubed preputial flap | 37 | 30 (81%) | ||
| Staged BMG flap | 10 | 6 (60%) | ||
| Staged BMG + local flap | 15 | 13 (86.6%) | ||
| Entero-urethroplasty | 2 | 2 (100%) | ||
| Radial forearm free flap | 3 | 3 (100%) | ||
| Pedicled anterolateral thigh flap | 1 | 1 (100%) | ||
| Mundy et al. [473] | Entero-urethroplasty | 11 | NA | 7 (63.6%) |
BMG = buccal mucosa graft; EPA = excision and primary anastomosis; N = number of patients; NA = not applicable.
| Summary of evidence | LE |
| The best results are obtained after the 1st urethroplasty. | 4 |
| The overall stricture-free rate after EPA is 85.7%. By using the progressive perineal approach, a combined transpubic abdomino-perineal approach is usually not needed. | 3 |
| After failed endoluminal treatment, EPA is the standard treatment for a non-obliterative stenosis. | 3 |
| Both a midline and inverted U perineal incision equally gain access to the posterior urethra, but a midline incision is associated with less anatomical damage to local vessels and nerves, reduced risk of surgical site infection and hospital stay. | 2b |
| Total pubectomy during transpubic abdomino-perineal reconstruction has a higher complication rate (bleeding, pelvic instability, dead space) compared to partial (superior or inferior) pubectomy with no gain in surgical exposure. | 4 |
| By using the progressive perineal approach, a combined transpubic abdomino-perineal approach is usually not needed except for very long distraction defects and in case of complicated situations, which include associated para-urethral bladder base fistula, trauma-related recto-urethral fistula, and bladder neck injury. | 3 |
| If the urethra cannot be anastomosed in a tension-free fashion or in case of ischaemic narrowing/necrosis of the bulbar urethra, options are a tubed preputial island flap, staged buccal mucosa graft urethroplasty with flap, staged buccal mucosa dartos flap, radial forearm free flap urethroplasty or entero-urethroplasty. | 3 |
| In case of excessive dead space after resection of the fibrosis, local flaps have been advised to fill up this space and support the anastomosis. These flaps are also useful to separate the suture lines in case of a concomitant recto-urethral fistula. | 3 |
| Recommendations | Strength rating |
| Perform open reconstruction for post-traumatic posterior stenosis only in high-volume centres. | Weak |
| Perform progressive perineal excision and primary anastomosis (EPA) for obliterative stenosis. | Strong |
| Perform progressive perineal EPA for non-obliterative stenosis after failed endoluminal treatment. | Strong |
| Perform a midline perineal incision to gain access to the posterior urethra. | Strong |
| Do not perform total pubectomy during abdomino-perineal reconstruction. | Strong |
| Reserve abdomino-perineal reconstruction for complicated situations including very long distraction defect, para-urethral bladder base fistula, trauma-related recto-urethral fistula, and bladder neck injury. | Weak |
| Perform another urethroplasty after 1st failed urethroplasty in motivated patients not willing to accept palliative endoluminal treatments or urinary diversion. | Weak |
| Use a local tissue flap to fill up excessive dead space or after correction of a concomitant recto-urethral fistula. | Weak |